 Found 82 hits Enz. Inhib. hit(s) with all data for entry = 50027629
Found 82 hits Enz. Inhib. hit(s) with all data for entry = 50027629 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254851
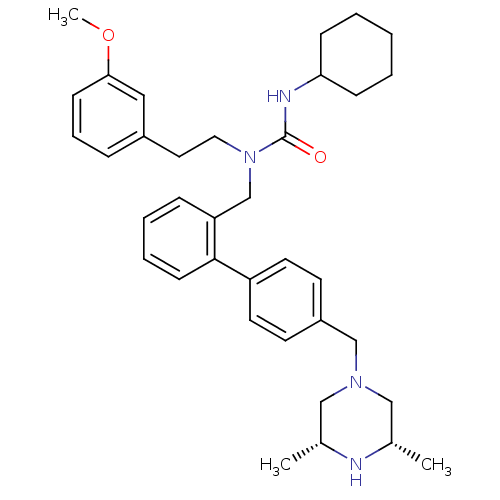
(3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3C[C@H](C)N[C@H](C)C3)cc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C36H48N4O2/c1-27-23-39(24-28(2)37-27)25-30-16-18-31(19-17-30)35-15-8-7-11-32(35)26-40(36(41)38-33-12-5-4-6-13-33)21-20-29-10-9-14-34(22-29)42-3/h7-11,14-19,22,27-28,33,37H,4-6,12-13,20-21,23-26H2,1-3H3,(H,38,41)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254851

(3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3C[C@H](C)N[C@H](C)C3)cc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C36H48N4O2/c1-27-23-39(24-28(2)37-27)25-30-16-18-31(19-17-30)35-15-8-7-11-32(35)26-40(36(41)38-33-12-5-4-6-13-33)21-20-29-10-9-14-34(22-29)42-3/h7-11,14-19,22,27-28,33,37H,4-6,12-13,20-21,23-26H2,1-3H3,(H,38,41)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254968
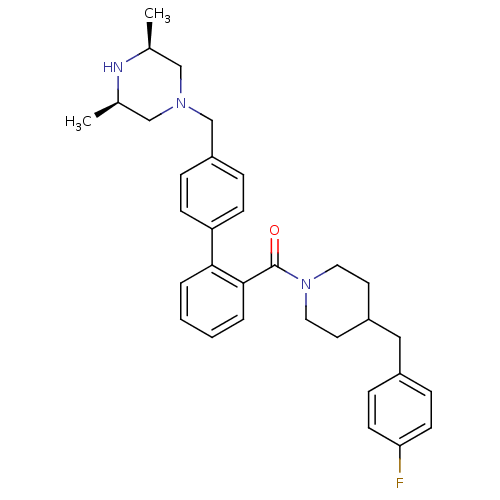
(CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(Cc3ccc(F)cc3)CC2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C32H38FN3O/c1-23-20-35(21-24(2)34-23)22-27-7-11-28(12-8-27)30-5-3-4-6-31(30)32(37)36-17-15-26(16-18-36)19-25-9-13-29(33)14-10-25/h3-14,23-24,26,34H,15-22H2,1-2H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254968

(CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(Cc3ccc(F)cc3)CC2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C32H38FN3O/c1-23-20-35(21-24(2)34-23)22-27-7-11-28(12-8-27)30-5-3-4-6-31(30)32(37)36-17-15-26(16-18-36)19-25-9-13-29(33)14-10-25/h3-14,23-24,26,34H,15-22H2,1-2H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254931
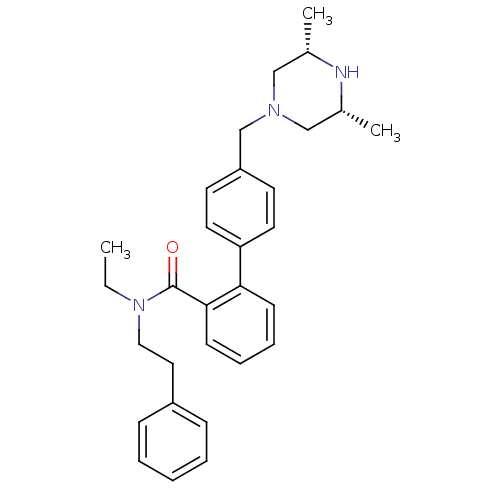
(4'-((3R,5S)-3,5-Dimethyl-piperazin-1-ylmethyl)-bip...)Show SMILES CCN(CCc1ccccc1)C(=O)c1ccccc1-c1ccc(CN2C[C@H](C)N[C@H](C)C2)cc1 |r| Show InChI InChI=1S/C30H37N3O/c1-4-33(19-18-25-10-6-5-7-11-25)30(34)29-13-9-8-12-28(29)27-16-14-26(15-17-27)22-32-20-23(2)31-24(3)21-32/h5-17,23-24,31H,4,18-22H2,1-3H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254892
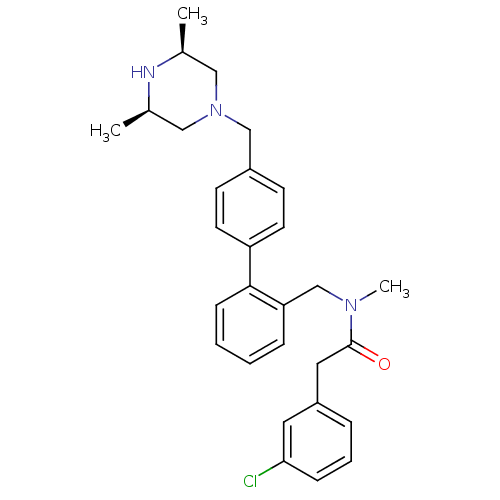
(2-(3-Chloro-phenyl)-N-[4'-((3R,5S)-3,5-dimethyl-pi...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2cccc(Cl)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-21-17-33(18-22(2)31-21)19-23-11-13-25(14-12-23)28-10-5-4-8-26(28)20-32(3)29(34)16-24-7-6-9-27(30)15-24/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254892

(2-(3-Chloro-phenyl)-N-[4'-((3R,5S)-3,5-dimethyl-pi...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2cccc(Cl)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-21-17-33(18-22(2)31-21)19-23-11-13-25(14-12-23)28-10-5-4-8-26(28)20-32(3)29(34)16-24-7-6-9-27(30)15-24/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254931

(4'-((3R,5S)-3,5-Dimethyl-piperazin-1-ylmethyl)-bip...)Show SMILES CCN(CCc1ccccc1)C(=O)c1ccccc1-c1ccc(CN2C[C@H](C)N[C@H](C)C2)cc1 |r| Show InChI InChI=1S/C30H37N3O/c1-4-33(19-18-25-10-6-5-7-11-25)30(34)29-13-9-8-12-28(29)27-16-14-26(15-17-27)22-32-20-23(2)31-24(3)21-32/h5-17,23-24,31H,4,18-22H2,1-3H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254893
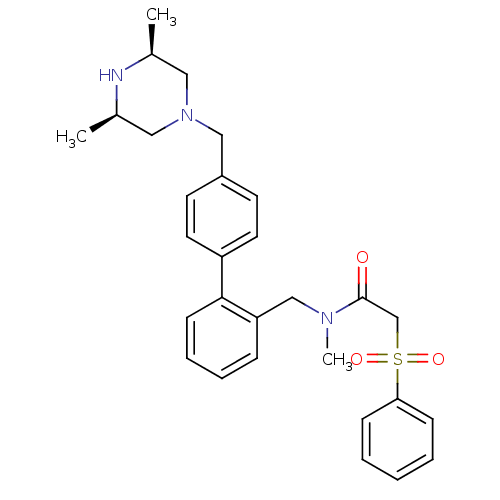
(2-Benzenesulfonyl-N-[4'-((3R,5S)-3,5-dimethyl-pipe...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)CS(=O)(=O)c2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H35N3O3S/c1-22-17-32(18-23(2)30-22)19-24-13-15-25(16-14-24)28-12-8-7-9-26(28)20-31(3)29(33)21-36(34,35)27-10-5-4-6-11-27/h4-16,22-23,30H,17-21H2,1-3H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254971
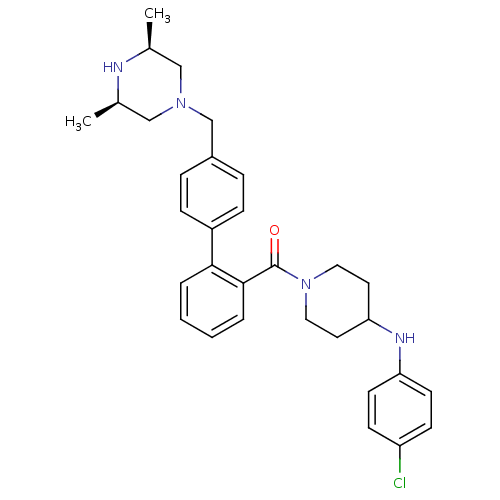
(CHEMBL481575 | [4-(4-Chloro-phenylamino)-piperidin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)Nc2ccc(Cl)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H37ClN4O/c1-22-19-35(20-23(2)33-22)21-24-7-9-25(10-8-24)29-5-3-4-6-30(29)31(37)36-17-15-28(16-18-36)34-27-13-11-26(32)12-14-27/h3-14,22-23,28,33-34H,15-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254999
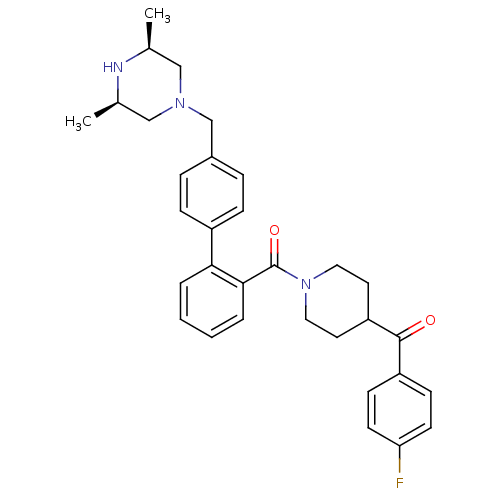
(CHEMBL480210 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)C(=O)c2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C32H36FN3O2/c1-22-19-35(20-23(2)34-22)21-24-7-9-25(10-8-24)29-5-3-4-6-30(29)32(38)36-17-15-27(16-18-36)31(37)26-11-13-28(33)14-12-26/h3-14,22-23,27,34H,15-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254999

(CHEMBL480210 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)C(=O)c2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C32H36FN3O2/c1-22-19-35(20-23(2)34-22)21-24-7-9-25(10-8-24)29-5-3-4-6-30(29)32(38)36-17-15-27(16-18-36)31(37)26-11-13-28(33)14-12-26/h3-14,22-23,27,34H,15-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254971

(CHEMBL481575 | [4-(4-Chloro-phenylamino)-piperidin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)Nc2ccc(Cl)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H37ClN4O/c1-22-19-35(20-23(2)33-22)21-24-7-9-25(10-8-24)29-5-3-4-6-30(29)31(37)36-17-15-28(16-18-36)34-27-13-11-26(32)12-14-27/h3-14,22-23,28,33-34H,15-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50255062
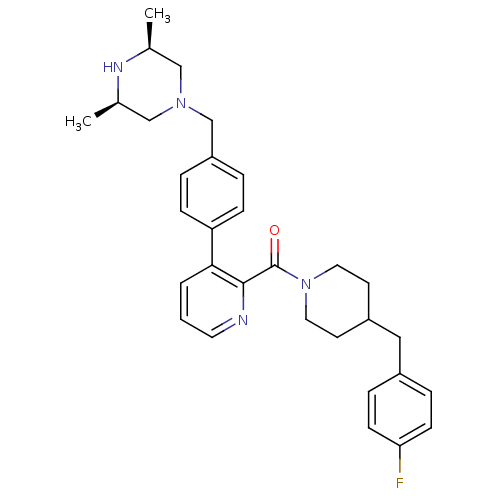
(CHEMBL465925 | {3-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(Cc3ccc(F)cc3)CC2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H37FN4O/c1-22-19-35(20-23(2)34-22)21-26-5-9-27(10-6-26)29-4-3-15-33-30(29)31(37)36-16-13-25(14-17-36)18-24-7-11-28(32)12-8-24/h3-12,15,22-23,25,34H,13-14,16-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254853
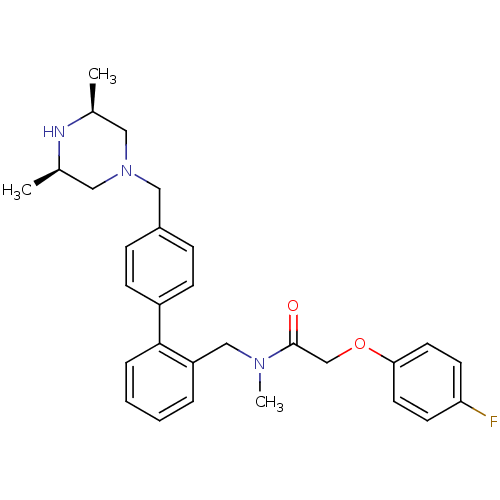
(CHEMBL464909 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)COc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34FN3O2/c1-21-16-33(17-22(2)31-21)18-23-8-10-24(11-9-23)28-7-5-4-6-25(28)19-32(3)29(34)20-35-27-14-12-26(30)13-15-27/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254852
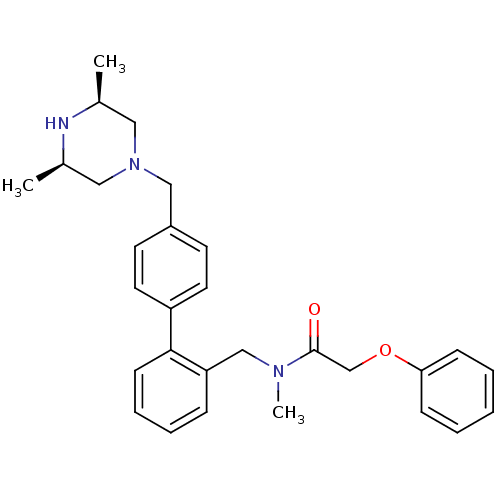
(CHEMBL465244 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)COc2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H35N3O2/c1-22-17-32(18-23(2)30-22)19-24-13-15-25(16-14-24)28-12-8-7-9-26(28)20-31(3)29(33)21-34-27-10-5-4-6-11-27/h4-16,22-23,30H,17-21H2,1-3H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254853

(CHEMBL464909 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)COc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34FN3O2/c1-21-16-33(17-22(2)31-21)18-23-8-10-24(11-9-23)28-7-5-4-6-25(28)19-32(3)29(34)20-35-27-14-12-26(30)13-15-27/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254969
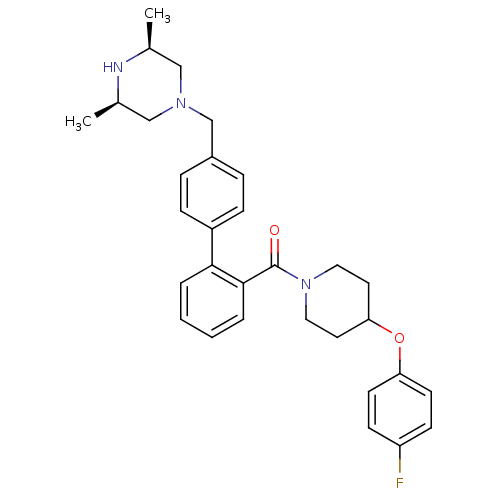
(CHEMBL518142 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)Oc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H36FN3O2/c1-22-19-34(20-23(2)33-22)21-24-7-9-25(10-8-24)29-5-3-4-6-30(29)31(36)35-17-15-28(16-18-35)37-27-13-11-26(32)12-14-27/h3-14,22-23,28,33H,15-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254893

(2-Benzenesulfonyl-N-[4'-((3R,5S)-3,5-dimethyl-pipe...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)CS(=O)(=O)c2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H35N3O3S/c1-22-17-32(18-23(2)30-22)19-24-13-15-25(16-14-24)28-12-8-7-9-26(28)20-31(3)29(33)21-36(34,35)27-10-5-4-6-11-27/h4-16,22-23,30H,17-21H2,1-3H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254970
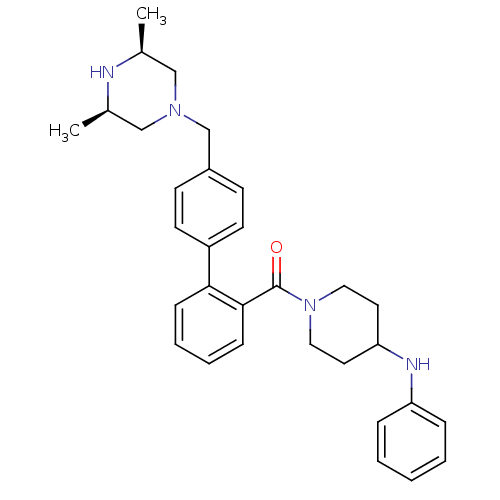
(CHEMBL479419 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)Nc2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H38N4O/c1-23-20-34(21-24(2)32-23)22-25-12-14-26(15-13-25)29-10-6-7-11-30(29)31(36)35-18-16-28(17-19-35)33-27-8-4-3-5-9-27/h3-15,23-24,28,32-33H,16-22H2,1-2H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50255062

(CHEMBL465925 | {3-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(Cc3ccc(F)cc3)CC2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H37FN4O/c1-22-19-35(20-23(2)34-22)21-26-5-9-27(10-6-26)29-4-3-15-33-30(29)31(37)36-16-13-25(14-17-36)18-24-7-11-28(32)12-8-24/h3-12,15,22-23,25,34H,13-14,16-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254969

(CHEMBL518142 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)Oc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H36FN3O2/c1-22-19-34(20-23(2)33-22)21-24-7-9-25(10-8-24)29-5-3-4-6-30(29)31(36)35-17-15-28(16-18-35)37-27-13-11-26(32)12-14-27/h3-14,22-23,28,33H,15-21H2,1-2H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50247157
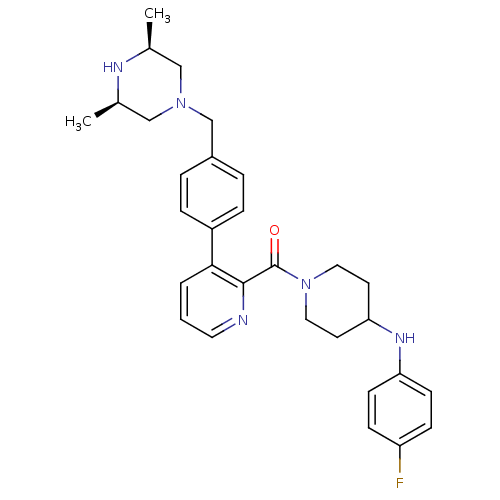
((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254970

(CHEMBL479419 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2C(=O)N2CCC(CC2)Nc2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C31H38N4O/c1-23-20-34(21-24(2)32-23)22-25-12-14-26(15-13-25)29-10-6-7-11-30(29)31(36)35-18-16-28(17-19-35)33-27-8-4-3-5-9-27/h3-15,23-24,28,32-33H,16-22H2,1-2H3/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254852

(CHEMBL465244 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)COc2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H35N3O2/c1-22-17-32(18-23(2)30-22)19-24-13-15-25(16-14-24)28-12-8-7-9-26(28)20-31(3)29(33)21-34-27-10-5-4-6-11-27/h4-16,22-23,30H,17-21H2,1-3H3/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50255029
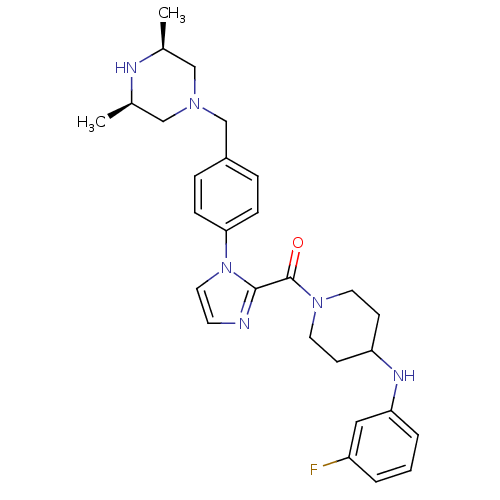
(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG binding |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG binding |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254930

(CHEMBL516551 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2cc(C)no2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C27H34N4O2/c1-19-13-25(33-29-19)14-27(32)30(4)18-24-7-5-6-8-26(24)23-11-9-22(10-12-23)17-31-15-20(2)28-21(3)16-31/h5-13,20-21,28H,14-18H2,1-4H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254930

(CHEMBL516551 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2cc(C)no2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C27H34N4O2/c1-19-13-25(33-29-19)14-27(32)30(4)18-24-7-5-6-8-26(24)23-11-9-22(10-12-23)17-31-15-20(2)28-21(3)16-31/h5-13,20-21,28H,14-18H2,1-4H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50412520
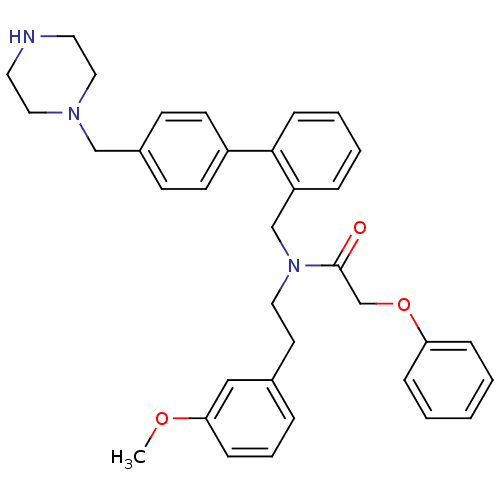
(CHEMBL14581)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3CCNCC3)cc2)C(=O)COc2ccccc2)c1 Show InChI InChI=1S/C35H39N3O3/c1-40-33-12-7-8-28(24-33)18-21-38(35(39)27-41-32-10-3-2-4-11-32)26-31-9-5-6-13-34(31)30-16-14-29(15-17-30)25-37-22-19-36-20-23-37/h2-17,24,36H,18-23,25-27H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50412521

(CHEMBL465104)Show SMILES COCCCN(Cc1ccccc1-c1ccc(CN2CCNCC2)cc1)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H37N3O3/c1-35-21-7-18-33(30(34)24-36-28-9-3-2-4-10-28)23-27-8-5-6-11-29(27)26-14-12-25(13-15-26)22-32-19-16-31-17-20-32/h2-6,8-15,31H,7,16-24H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50254851

(3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3C[C@H](C)N[C@H](C)C3)cc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C36H48N4O2/c1-27-23-39(24-28(2)37-27)25-30-16-18-31(19-17-30)35-15-8-7-11-32(35)26-40(36(41)38-33-12-5-4-6-13-33)21-20-29-10-9-14-34(22-29)42-3/h7-11,14-19,22,27-28,33,37H,4-6,12-13,20-21,23-26H2,1-3H3,(H,38,41)/t27-,28+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in CHO cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50344942
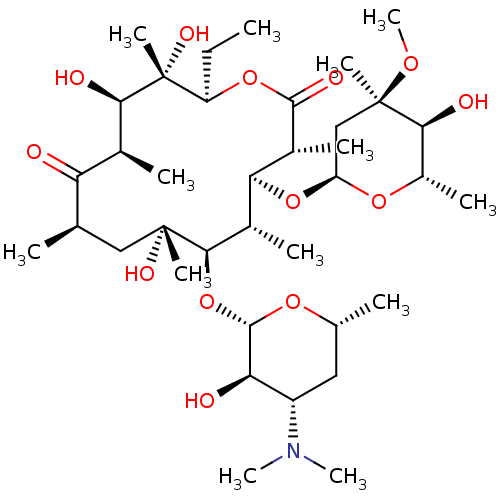
(CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r| Show InChI InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in CHO cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Ghrelin receptor |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50255029

(CHEMBL480219 | {1-[4-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-n2ccnc2C(=O)N2CCC(CC2)Nc2cccc(F)c2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H35FN6O/c1-20-17-33(18-21(2)31-20)19-22-6-8-26(9-7-22)35-15-12-30-27(35)28(36)34-13-10-24(11-14-34)32-25-5-3-4-23(29)16-25/h3-9,12,15-16,20-21,24,31-32H,10-11,13-14,17-19H2,1-2H3/t20-,21+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Ghrelin receptor |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50254851

(3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...)Show SMILES COc1cccc(CCN(Cc2ccccc2-c2ccc(CN3C[C@H](C)N[C@H](C)C3)cc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C36H48N4O2/c1-27-23-39(24-28(2)37-27)25-30-16-18-31(19-17-30)35-15-8-7-11-32(35)26-40(36(41)38-33-12-5-4-6-13-33)21-20-29-10-9-14-34(22-29)42-3/h7-11,14-19,22,27-28,33,37H,4-6,12-13,20-21,23-26H2,1-3H3,(H,38,41)/t27-,28+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50254852

(CHEMBL465244 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)COc2ccccc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H35N3O2/c1-22-17-32(18-23(2)30-22)19-24-13-15-25(16-14-24)28-12-8-7-9-26(28)20-31(3)29(33)21-34-27-10-5-4-6-11-27/h4-16,22-23,30H,17-21H2,1-3H3/t22-,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50254853

(CHEMBL464909 | N-[4'-((3R,5S)-3,5-Dimethyl-piperaz...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)COc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34FN3O2/c1-21-16-33(17-22(2)31-21)18-23-8-10-24(11-9-23)28-7-5-4-6-25(28)19-32(3)29(34)20-35-27-14-12-26(30)13-15-27/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50412522
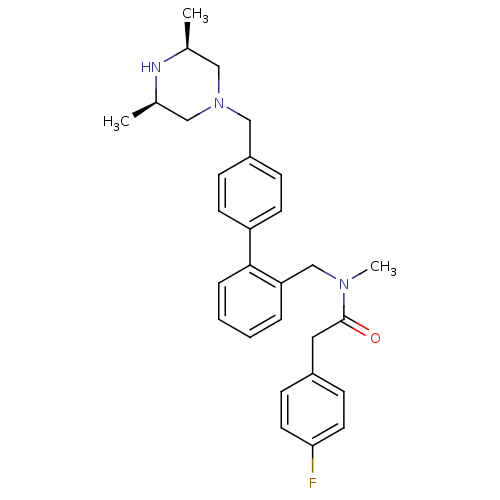
(CHEMBL465940)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2ccccc2CN(C)C(=O)Cc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C29H34FN3O/c1-21-17-33(18-22(2)31-21)19-24-8-12-25(13-9-24)28-7-5-4-6-26(28)20-32(3)29(34)16-23-10-14-27(30)15-11-23/h4-15,21-22,31H,16-20H2,1-3H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant motilin receptor expressed in HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 18: 6429-36 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.072
BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data