 Found 39 hits Enz. Inhib. hit(s) with all data for entry = 50046918
Found 39 hits Enz. Inhib. hit(s) with all data for entry = 50046918 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138119
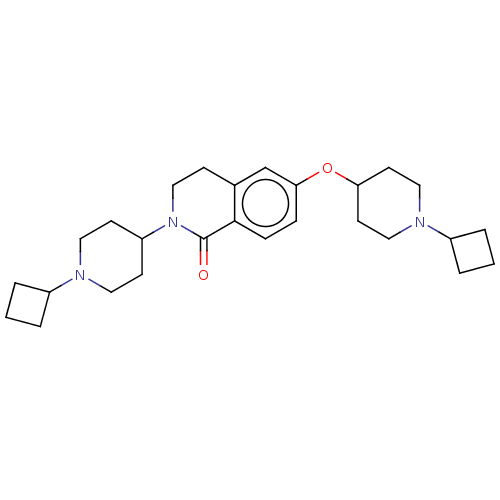
(CHEMBL3753475)Show SMILES O=C1N(CCc2cc(OC3CCN(CC3)C3CCC3)ccc12)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C27H39N3O2/c31-27-26-8-7-25(32-24-12-16-29(17-13-24)22-5-2-6-22)19-20(26)9-18-30(27)23-10-14-28(15-11-23)21-3-1-4-21/h7-8,19,21-24H,1-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138124
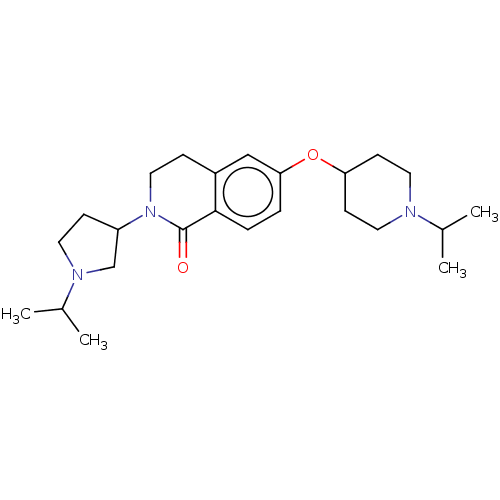
(CHEMBL3753901)Show SMILES CC(C)N1CCC(C1)N1CCc2cc(OC3CCN(CC3)C(C)C)ccc2C1=O Show InChI InChI=1S/C24H37N3O2/c1-17(2)25-12-9-21(10-13-25)29-22-5-6-23-19(15-22)7-14-27(24(23)28)20-8-11-26(16-20)18(3)4/h5-6,15,17-18,20-21H,7-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138119

(CHEMBL3753475)Show SMILES O=C1N(CCc2cc(OC3CCN(CC3)C3CCC3)ccc12)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C27H39N3O2/c31-27-26-8-7-25(32-24-12-16-29(17-13-24)22-5-2-6-22)19-20(26)9-18-30(27)23-10-14-28(15-11-23)21-3-1-4-21/h7-8,19,21-24H,1-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138120
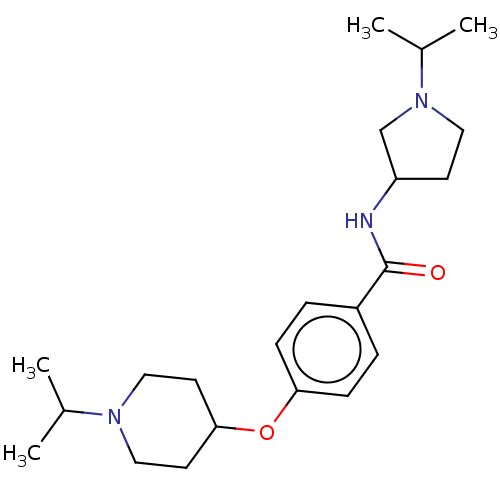
(CHEMBL3753093)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C22H35N3O2/c1-16(2)24-13-10-21(11-14-24)27-20-7-5-18(6-8-20)22(26)23-19-9-12-25(15-19)17(3)4/h5-8,16-17,19,21H,9-15H2,1-4H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138123

(CHEMBL3753703)Show SMILES CC(C)CN1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C23H37N3O2/c1-17(2)15-25-12-9-20(16-25)24-23(27)19-5-7-21(8-6-19)28-22-10-13-26(14-11-22)18(3)4/h5-8,17-18,20,22H,9-16H2,1-4H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138122
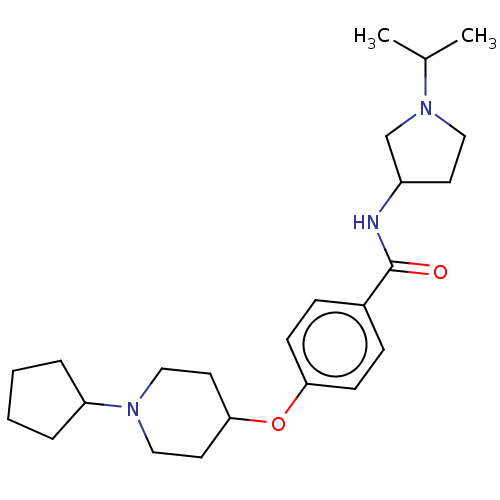
(CHEMBL3752655)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C24H37N3O2/c1-18(2)27-14-11-20(17-27)25-24(28)19-7-9-22(10-8-19)29-23-12-15-26(16-13-23)21-5-3-4-6-21/h7-10,18,20-21,23H,3-6,11-17H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138123

(CHEMBL3753703)Show SMILES CC(C)CN1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C23H37N3O2/c1-17(2)15-25-12-9-20(16-25)24-23(27)19-5-7-21(8-6-19)28-22-10-13-26(14-11-22)18(3)4/h5-8,17-18,20,22H,9-16H2,1-4H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138117
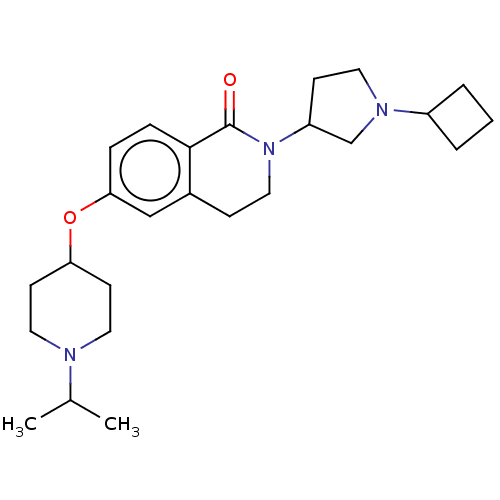
(CHEMBL3753315)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2C(=O)N(CCc2c1)C1CCN(C1)C1CCC1 Show InChI InChI=1S/C25H37N3O2/c1-18(2)26-13-10-22(11-14-26)30-23-6-7-24-19(16-23)8-15-28(25(24)29)21-9-12-27(17-21)20-4-3-5-20/h6-7,16,18,20-22H,3-5,8-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138106
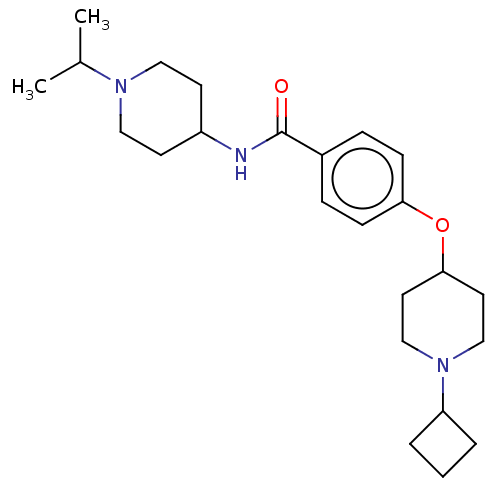
(CHEMBL3753814)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H37N3O2/c1-18(2)26-14-10-20(11-15-26)25-24(28)19-6-8-22(9-7-19)29-23-12-16-27(17-13-23)21-4-3-5-21/h6-9,18,20-21,23H,3-5,10-17H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138118
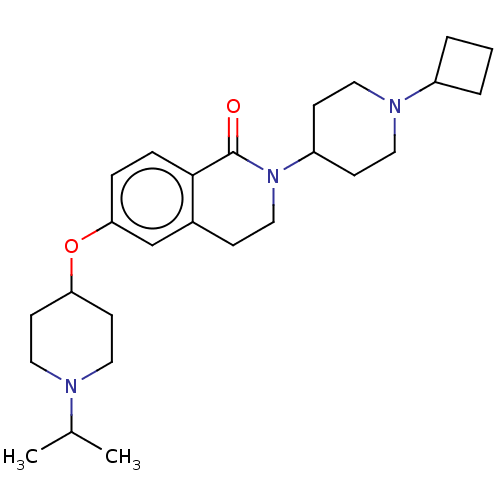
(CHEMBL3754200)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2C(=O)N(CCc2c1)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C26H39N3O2/c1-19(2)27-15-11-23(12-16-27)31-24-6-7-25-20(18-24)8-17-29(26(25)30)22-9-13-28(14-10-22)21-4-3-5-21/h6-7,18-19,21-23H,3-5,8-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138124

(CHEMBL3753901)Show SMILES CC(C)N1CCC(C1)N1CCc2cc(OC3CCN(CC3)C(C)C)ccc2C1=O Show InChI InChI=1S/C24H37N3O2/c1-17(2)25-12-9-21(10-13-25)29-22-5-6-23-19(15-22)7-14-27(24(23)28)20-8-11-26(16-20)18(3)4/h5-6,15,17-18,20-21H,7-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138104
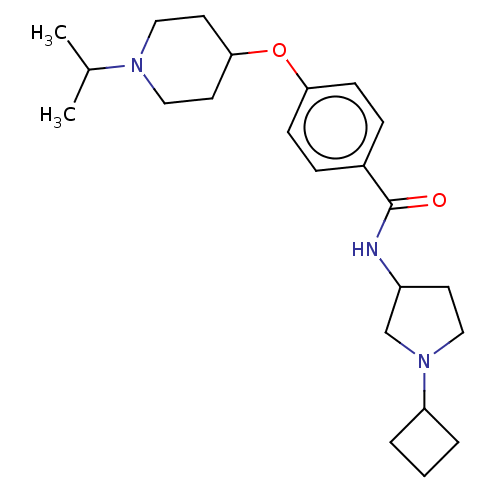
(CHEMBL3754209)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(cc1)C(=O)NC1CCN(C1)C1CCC1 Show InChI InChI=1S/C23H35N3O2/c1-17(2)25-14-11-22(12-15-25)28-21-8-6-18(7-9-21)23(27)24-19-10-13-26(16-19)20-4-3-5-20/h6-9,17,19-20,22H,3-5,10-16H2,1-2H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138125
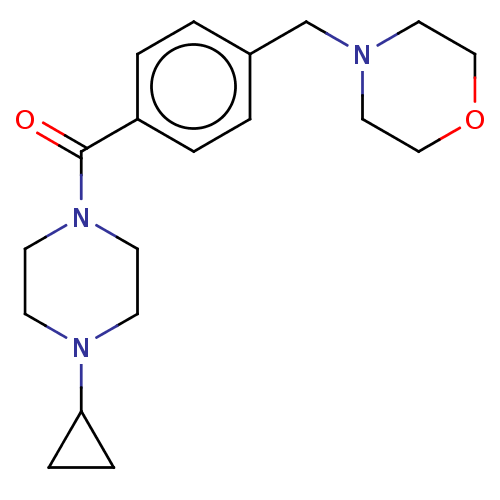
(Bavisant | JNJ-31001074)Show InChI InChI=1S/C19H27N3O2/c23-19(22-9-7-21(8-10-22)18-5-6-18)17-3-1-16(2-4-17)15-20-11-13-24-14-12-20/h1-4,18H,5-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138120

(CHEMBL3753093)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C22H35N3O2/c1-16(2)24-13-10-21(11-14-24)27-20-7-5-18(6-8-20)22(26)23-19-9-12-25(15-19)17(3)4/h5-8,16-17,19,21H,9-15H2,1-4H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138113
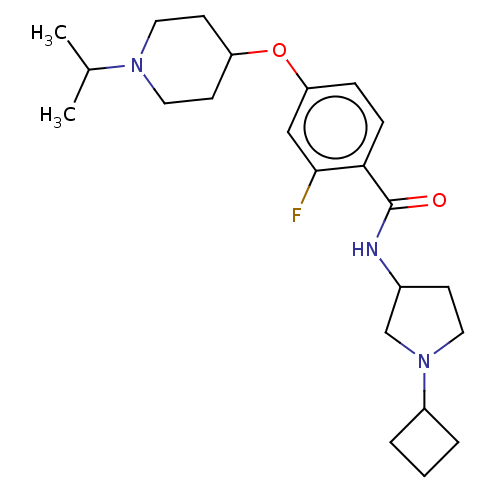
(CHEMBL3753170)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(C(=O)NC2CCN(C2)C2CCC2)c(F)c1 Show InChI InChI=1S/C23H34FN3O2/c1-16(2)26-12-9-19(10-13-26)29-20-6-7-21(22(24)14-20)23(28)25-17-8-11-27(15-17)18-4-3-5-18/h6-7,14,16-19H,3-5,8-13,15H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138112
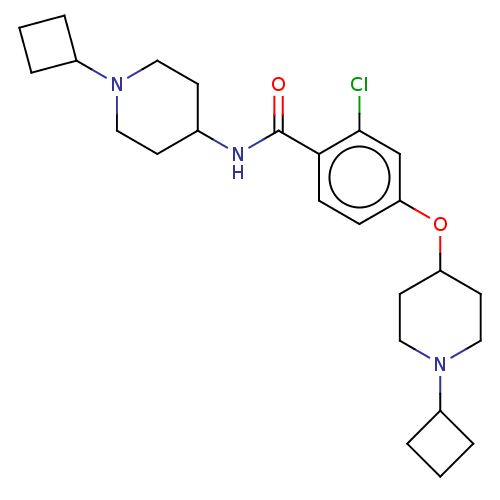
(CHEMBL3753147)Show SMILES Clc1cc(OC2CCN(CC2)C2CCC2)ccc1C(=O)NC1CCN(CC1)C1CCC1 Show InChI InChI=1S/C25H36ClN3O2/c26-24-17-22(31-21-11-15-29(16-12-21)20-5-2-6-20)7-8-23(24)25(30)27-18-9-13-28(14-10-18)19-3-1-4-19/h7-8,17-21H,1-6,9-16H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50262939
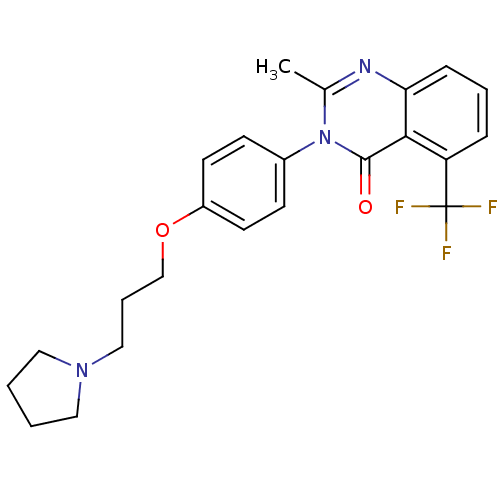
(2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...)Show SMILES Cc1nc2cccc(c2c(=O)n1-c1ccc(OCCCN2CCCC2)cc1)C(F)(F)F Show InChI InChI=1S/C23H24F3N3O2/c1-16-27-20-7-4-6-19(23(24,25)26)21(20)22(30)29(16)17-8-10-18(11-9-17)31-15-5-14-28-12-2-3-13-28/h4,6-11H,2-3,5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138111
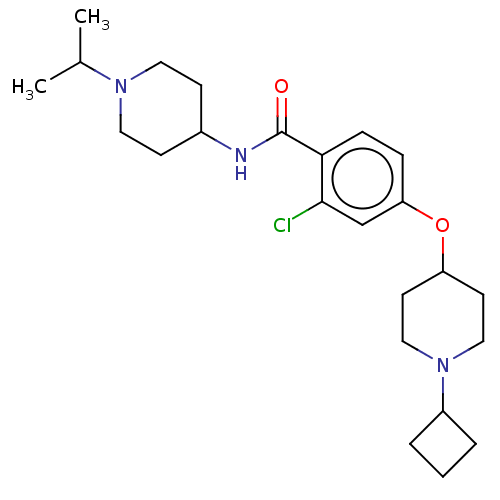
(CHEMBL3751915)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1Cl Show InChI InChI=1S/C24H36ClN3O2/c1-17(2)27-12-8-18(9-13-27)26-24(29)22-7-6-21(16-23(22)25)30-20-10-14-28(15-11-20)19-4-3-5-19/h6-7,16-20H,3-5,8-15H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138107
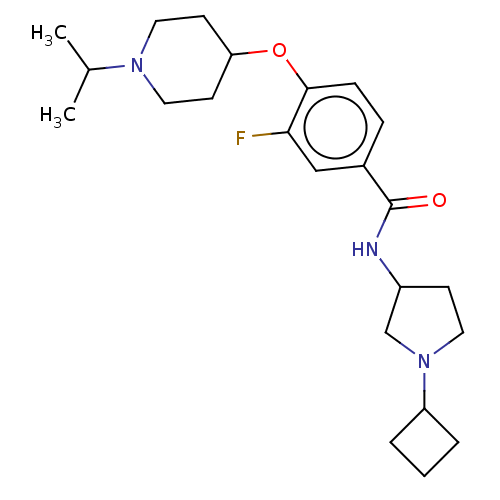
(CHEMBL3753304)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(cc1F)C(=O)NC1CCN(C1)C1CCC1 Show InChI InChI=1S/C23H34FN3O2/c1-16(2)26-12-9-20(10-13-26)29-22-7-6-17(14-21(22)24)23(28)25-18-8-11-27(15-18)19-4-3-5-19/h6-7,14,16,18-20H,3-5,8-13,15H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138105

(CHEMBL3752081)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C23H37N3O2/c1-17(2)25-13-9-20(10-14-25)24-23(27)19-5-7-21(8-6-19)28-22-11-15-26(16-12-22)18(3)4/h5-8,17-18,20,22H,9-16H2,1-4H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138122

(CHEMBL3752655)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C24H37N3O2/c1-18(2)27-14-11-20(17-27)25-24(28)19-7-9-22(10-8-19)29-23-12-15-26(16-13-23)21-5-3-4-6-21/h7-10,18,20-21,23H,3-6,11-17H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138114

(CHEMBL3753764)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(C(=O)NC2CCN(C2)C2CCC2)c(C)c1 Show InChI InChI=1S/C24H37N3O2/c1-17(2)26-13-10-21(11-14-26)29-22-7-8-23(18(3)15-22)24(28)25-19-9-12-27(16-19)20-5-4-6-20/h7-8,15,17,19-21H,4-6,9-14,16H2,1-3H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50444496
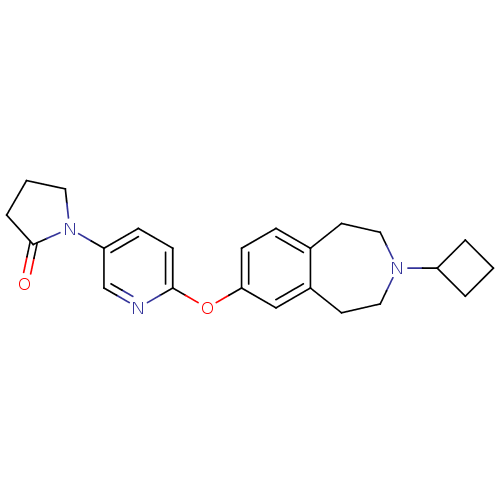
(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138109
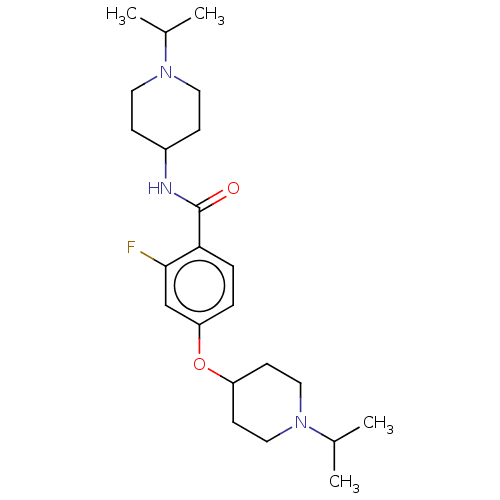
(CHEMBL3752705)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1F Show InChI InChI=1S/C23H36FN3O2/c1-16(2)26-11-7-18(8-12-26)25-23(28)21-6-5-20(15-22(21)24)29-19-9-13-27(14-10-19)17(3)4/h5-6,15-19H,7-14H2,1-4H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138108
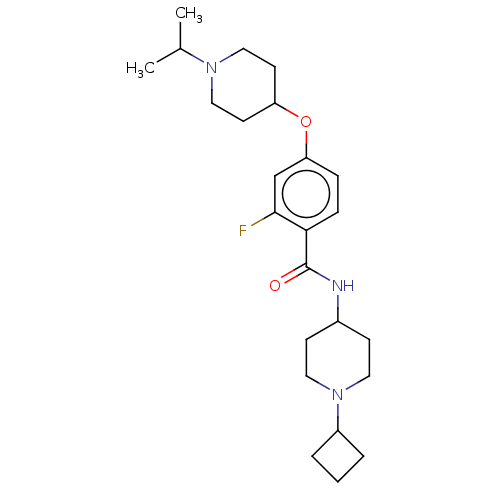
(CHEMBL3753102)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(C(=O)NC2CCN(CC2)C2CCC2)c(F)c1 Show InChI InChI=1S/C24H36FN3O2/c1-17(2)27-14-10-20(11-15-27)30-21-6-7-22(23(25)16-21)24(29)26-18-8-12-28(13-9-18)19-4-3-5-19/h6-7,16-20H,3-5,8-15H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138110

(CHEMBL3752796)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(C(=O)NC2CCN(C2)C2CCC2)c(Cl)c1 Show InChI InChI=1S/C23H34ClN3O2/c1-16(2)26-12-9-19(10-13-26)29-20-6-7-21(22(24)14-20)23(28)25-17-8-11-27(15-17)18-4-3-5-18/h6-7,14,16-19H,3-5,8-13,15H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138121
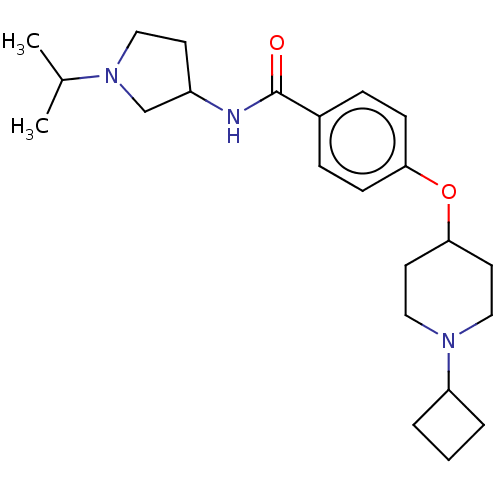
(CHEMBL3754205)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C23H35N3O2/c1-17(2)26-13-10-19(16-26)24-23(27)18-6-8-21(9-7-18)28-22-11-14-25(15-12-22)20-4-3-5-20/h6-9,17,19-20,22H,3-5,10-16H2,1-2H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138103
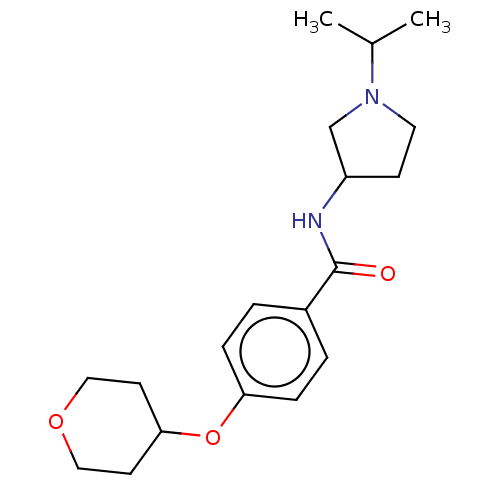
(CHEMBL3752696)Show InChI InChI=1S/C19H28N2O3/c1-14(2)21-10-7-16(13-21)20-19(22)15-3-5-17(6-4-15)24-18-8-11-23-12-9-18/h3-6,14,16,18H,7-13H2,1-2H3,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138116
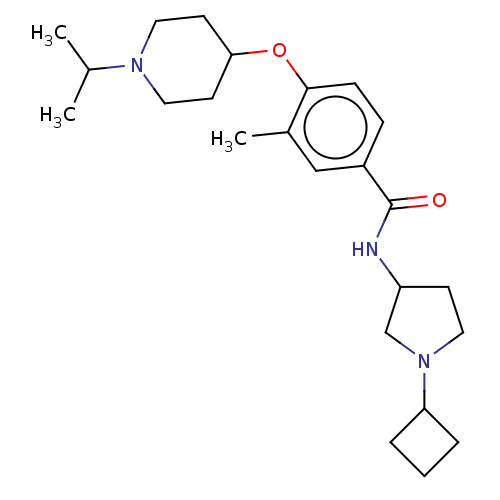
(CHEMBL3754674)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(cc1C)C(=O)NC1CCN(C1)C1CCC1 Show InChI InChI=1S/C24H37N3O2/c1-17(2)26-13-10-22(11-14-26)29-23-8-7-19(15-18(23)3)24(28)25-20-9-12-27(16-20)21-5-4-6-21/h7-8,15,17,20-22H,4-6,9-14,16H2,1-3H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138115

(CHEMBL3754726)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)c(C)c1 Show InChI InChI=1S/C23H37N3O2/c1-16(2)25-12-9-21(10-13-25)28-22-7-6-19(14-18(22)5)23(27)24-20-8-11-26(15-20)17(3)4/h6-7,14,16-17,20-21H,8-13,15H2,1-5H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-alpha-methyl histamine from recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138119

(CHEMBL3753475)Show SMILES O=C1N(CCc2cc(OC3CCN(CC3)C3CCC3)ccc12)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C27H39N3O2/c31-27-26-8-7-25(32-24-12-16-29(17-13-24)22-5-2-6-22)19-20(26)9-18-30(27)23-10-14-28(15-11-23)21-3-1-4-21/h7-8,19,21-24H,1-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed on CHO-K1 cells assessed as cAMP level by luciferase gene reporter assay |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138124

(CHEMBL3753901)Show SMILES CC(C)N1CCC(C1)N1CCc2cc(OC3CCN(CC3)C(C)C)ccc2C1=O Show InChI InChI=1S/C24H37N3O2/c1-17(2)25-12-9-21(10-13-25)29-22-5-6-23-19(15-22)7-14-27(24(23)28)20-8-11-26(16-20)18(3)4/h5-6,15,17-18,20-21H,7-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed on CHO-K1 cells assessed as cAMP level by luciferase gene reporter assay |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138122

(CHEMBL3752655)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C24H37N3O2/c1-18(2)27-14-11-20(17-27)25-24(28)19-7-9-22(10-8-19)29-23-12-15-26(16-13-23)21-5-3-4-6-21/h7-10,18,20-21,23H,3-6,11-17H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed on CHO-K1 cells assessed as cAMP level by luciferase gene reporter assay |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138120

(CHEMBL3753093)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C22H35N3O2/c1-16(2)24-13-10-21(11-14-24)27-20-7-5-18(6-8-20)22(26)23-19-9-12-25(15-19)17(3)4/h5-8,16-17,19,21H,9-15H2,1-4H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed on CHO-K1 cells assessed as cAMP level by luciferase gene reporter assay |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138123

(CHEMBL3753703)Show SMILES CC(C)CN1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C23H37N3O2/c1-17(2)15-25-12-9-20(16-25)24-23(27)19-5-7-21(8-6-19)28-22-10-13-26(14-11-22)18(3)4/h5-8,17-18,20,22H,9-16H2,1-4H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed on CHO-K1 cells assessed as cAMP level by luciferase gene reporter assay |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138121

(CHEMBL3754205)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C23H35N3O2/c1-17(2)26-13-10-19(16-26)24-23(27)18-6-8-21(9-7-18)28-22-11-14-25(15-12-22)20-4-3-5-20/h6-9,17,19-20,22H,3-5,10-16H2,1-2H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 12 mins by LC-MS/MS analysis |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138121

(CHEMBL3754205)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C23H35N3O2/c1-17(2)26-13-10-19(16-26)24-23(27)18-6-8-21(9-7-18)28-22-11-14-25(15-12-22)20-4-3-5-20/h6-9,17,19-20,22H,3-5,10-16H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 2 mins by LC-MS/MS analysis |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138120

(CHEMBL3753093)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C22H35N3O2/c1-16(2)24-13-10-21(11-14-24)27-20-7-5-18(6-8-20)22(26)23-19-9-12-25(15-19)17(3)4/h5-8,16-17,19,21H,9-15H2,1-4H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 2 mins by LC-MS/MS analysis |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138120

(CHEMBL3753093)Show SMILES CC(C)N1CCC(C1)NC(=O)c1ccc(OC2CCN(CC2)C(C)C)cc1 Show InChI InChI=1S/C22H35N3O2/c1-16(2)24-13-10-21(11-14-24)27-20-7-5-18(6-8-20)22(26)23-19-9-12-25(15-19)17(3)4/h5-8,16-17,19,21H,9-15H2,1-4H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 12 mins by LC-MS/MS analysis |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data