 Found 22 hits Enz. Inhib. hit(s) with all data for entry = 50046927
Found 22 hits Enz. Inhib. hit(s) with all data for entry = 50046927 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138819
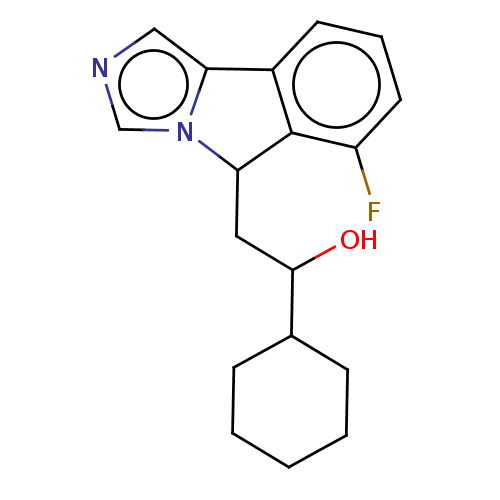
(CHEMBL3753837 | US10233190, Example 1357)Show InChI InChI=1S/C18H21FN2O/c19-14-8-4-7-13-16-10-20-11-21(16)15(18(13)14)9-17(22)12-5-2-1-3-6-12/h4,7-8,10-12,15,17,22H,1-3,5-6,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138818
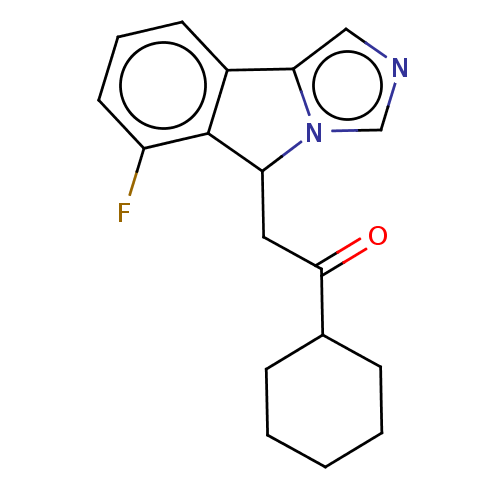
(CHEMBL3754460 | US10233190, Example 1356)Show InChI InChI=1S/C18H19FN2O/c19-14-8-4-7-13-16-10-20-11-21(16)15(18(13)14)9-17(22)12-5-2-1-3-6-12/h4,7-8,10-12,15H,1-3,5-6,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126145
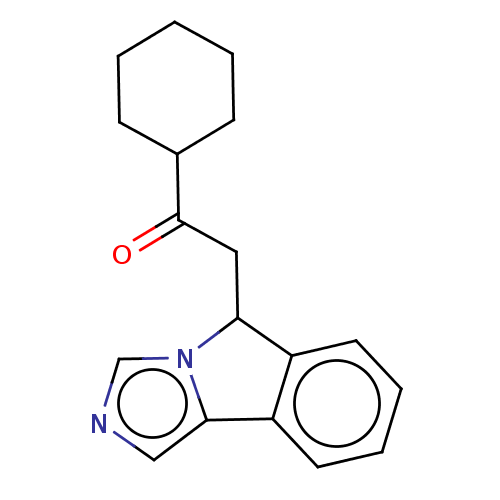
(CHEMBL3629570)Show InChI InChI=1S/C18H20N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138829
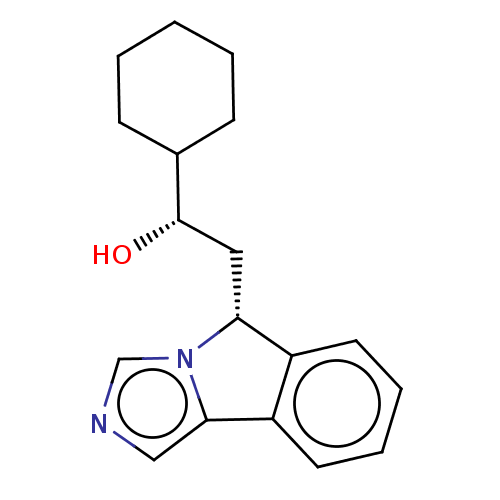
(CHEMBL3754097 | US10233190, Example 1416)Show SMILES O[C@@H](C[C@@H]1c2ccccc2-c2cncn12)C1CCCCC1 |r| Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in M15(pREP4) cells using L-tryptophan as substrate assessed as conversion of N-formylkynurenine to ky... |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138830

(CHEMBL3752659 | US10233190, Example 1415)Show SMILES O[C@H](C[C@@H]1c2ccccc2-c2cncn12)C1CCCCC1 |r| Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2/t16-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in M15(pREP4) cells using L-tryptophan as substrate assessed as conversion of N-formylkynurenine to ky... |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138828
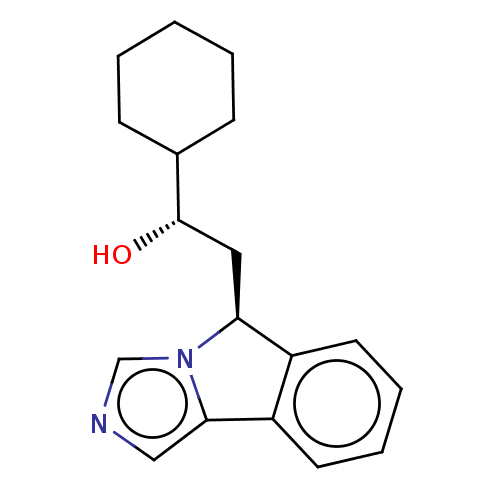
(CHEMBL3752060 | US10233190, Example 1417)Show SMILES O[C@@H](C[C@H]1c2ccccc2-c2cncn12)C1CCCCC1 |r| Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in M15(pREP4) cells using L-tryptophan as substrate assessed as conversion of N-formylkynurenine to ky... |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138827
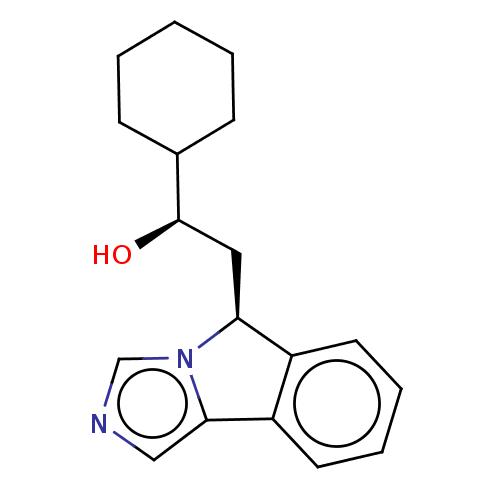
(CHEMBL3752711 | US10233190, Example 1418)Show SMILES O[C@H](C[C@H]1c2ccccc2-c2cncn12)C1CCCCC1 |r| Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2/t16-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in M15(pREP4) cells using L-tryptophan as substrate assessed as conversion of N-formylkynurenine to ky... |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138822
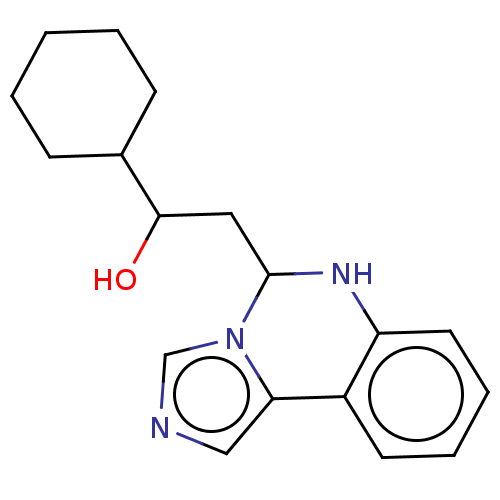
(CHEMBL3753777)Show InChI InChI=1S/C18H23N3O/c22-17(13-6-2-1-3-7-13)10-18-20-15-9-5-4-8-14(15)16-11-19-12-21(16)18/h4-5,8-9,11-13,17-18,20,22H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50030791
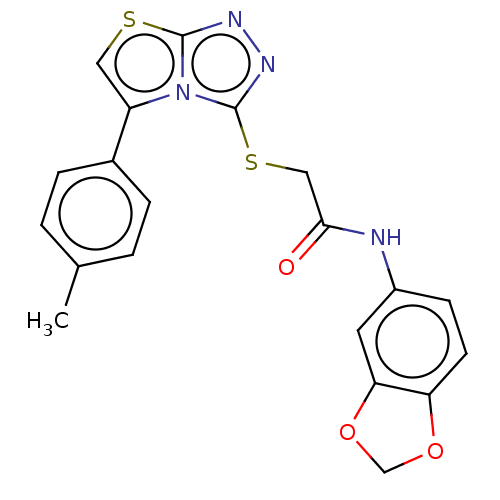
(CHEMBL3342402)Show SMILES Cc1ccc(cc1)-c1csc2nnc(SCC(=O)Nc3ccc4OCOc4c3)n12 Show InChI InChI=1S/C20H16N4O3S2/c1-12-2-4-13(5-3-12)15-9-28-19-22-23-20(24(15)19)29-10-18(25)21-14-6-7-16-17(8-14)27-11-26-16/h2-9H,10-11H2,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 by Bridge-IT Tryptophan fluorescence assay |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138821

(CHEMBL3747548 | US9617272, Compound 66 | US9981973...)Show InChI InChI=1S/C17H21N3O/c21-17(12-6-2-1-3-7-12)10-15-13-8-4-5-9-14(13)16-11-18-19-20(15)16/h4-5,8-9,11-12,15,17,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138826
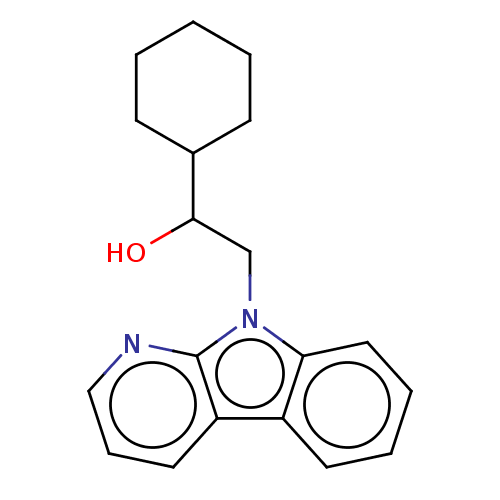
(CHEMBL3752732)Show InChI InChI=1S/C19H22N2O/c22-18(14-7-2-1-3-8-14)13-21-17-11-5-4-9-15(17)16-10-6-12-20-19(16)21/h4-6,9-12,14,18,22H,1-3,7-8,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138825

(CHEMBL3754016)Show InChI InChI=1S/C19H20N2O/c22-18(14-7-2-1-3-8-14)13-21-17-11-5-4-9-15(17)16-10-6-12-20-19(16)21/h4-6,9-12,14H,1-3,7-8,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138820
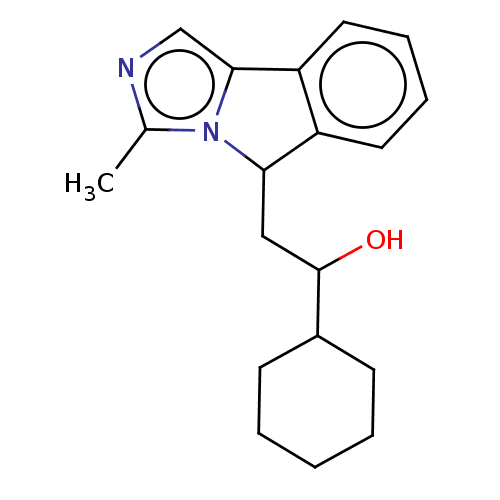
(CHEMBL3752239)Show InChI InChI=1S/C19H24N2O/c1-13-20-12-18-16-10-6-5-9-15(16)17(21(13)18)11-19(22)14-7-3-2-4-8-14/h5-6,9-10,12,14,17,19,22H,2-4,7-8,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138824
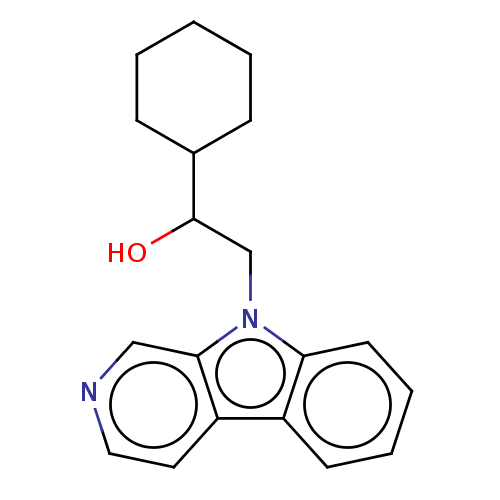
(CHEMBL3752209)Show InChI InChI=1S/C19H22N2O/c22-19(14-6-2-1-3-7-14)13-21-17-9-5-4-8-15(17)16-10-11-20-12-18(16)21/h4-5,8-12,14,19,22H,1-3,6-7,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138822

(CHEMBL3753777)Show InChI InChI=1S/C18H23N3O/c22-17(13-6-2-1-3-7-13)10-18-20-15-9-5-4-8-14(15)16-11-19-12-21(16)18/h4-5,8-9,11-13,17-18,20,22H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138821

(CHEMBL3747548 | US9617272, Compound 66 | US9981973...)Show InChI InChI=1S/C17H21N3O/c21-17(12-6-2-1-3-7-12)10-15-13-8-4-5-9-14(13)16-11-18-19-20(15)16/h4-5,8-9,11-12,15,17,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138819

(CHEMBL3753837 | US10233190, Example 1357)Show InChI InChI=1S/C18H21FN2O/c19-14-8-4-7-13-16-10-20-11-21(16)15(18(13)14)9-17(22)12-5-2-1-3-6-12/h4,7-8,10-12,15,17,22H,1-3,5-6,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138818

(CHEMBL3754460 | US10233190, Example 1356)Show InChI InChI=1S/C18H19FN2O/c19-14-8-4-7-13-16-10-20-11-21(16)15(18(13)14)9-17(22)12-5-2-1-3-6-12/h4,7-8,10-12,15H,1-3,5-6,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 725 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126145

(CHEMBL3629570)Show InChI InChI=1S/C18H20N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 749 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138820

(CHEMBL3752239)Show InChI InChI=1S/C19H24N2O/c1-13-20-12-18-16-10-6-5-9-15(16)17(21(13)18)11-19(22)14-7-3-2-4-8-14/h5-6,9-10,12,14,17,19,22H,2-4,7-8,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs |
J Med Chem 59: 282-93 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01390
BindingDB Entry DOI: 10.7270/Q28917QW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data