

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140041 (CHEMBL3765059) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140039 (CHEMBL3764556) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139929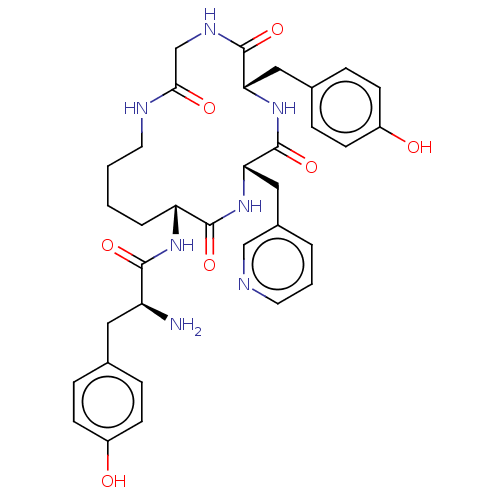 (CHEMBL3763920) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139926 (CHEMBL3764157) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139928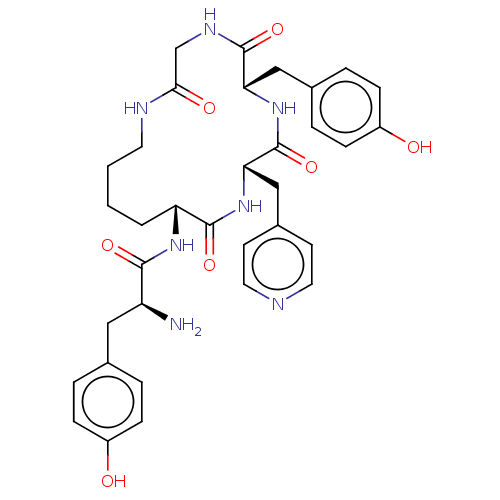 (CHEMBL3763985) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140042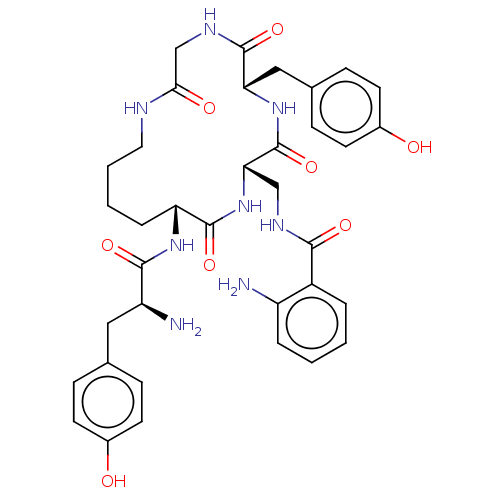 (CHEMBL3763495) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139927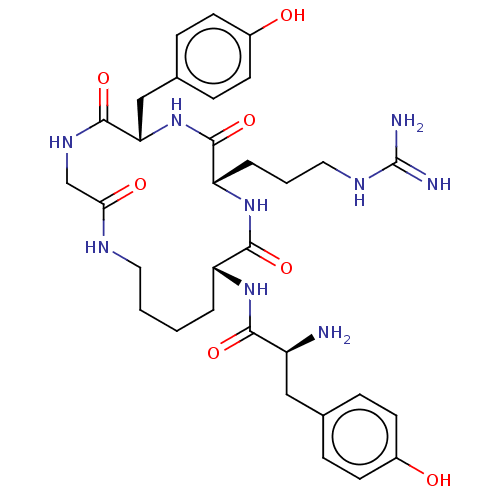 (CHEMBL3765741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140041 (CHEMBL3765059) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140039 (CHEMBL3764556) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140040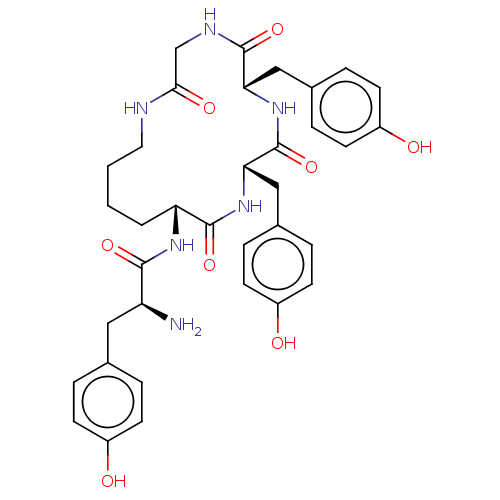 (CHEMBL3763905) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140040 (CHEMBL3763905) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140038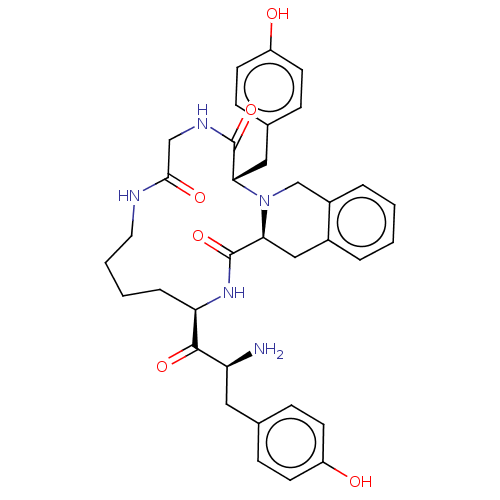 (CHEMBL3764234) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140037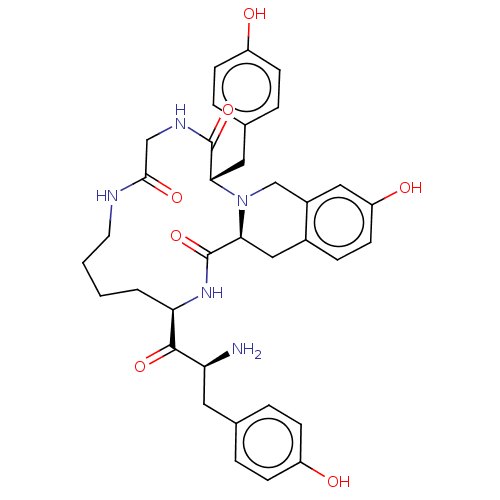 (CHEMBL3763452) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140042 (CHEMBL3763495) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140038 (CHEMBL3764234) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 991 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140037 (CHEMBL3763452) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139929 (CHEMBL3763920) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139928 (CHEMBL3763985) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50140042 (CHEMBL3763495) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139926 (CHEMBL3764157) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50140041 (CHEMBL3765059) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50139929 (CHEMBL3763920) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50140039 (CHEMBL3764556) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50139926 (CHEMBL3764157) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50139927 (CHEMBL3765741) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139927 (CHEMBL3765741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50140040 (CHEMBL3763905) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50140037 (CHEMBL3763452) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50139928 (CHEMBL3763985) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50140038 (CHEMBL3764234) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50140041 (CHEMBL3765059) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139929 (CHEMBL3763920) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50140039 (CHEMBL3764556) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139928 (CHEMBL3763985) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139926 (CHEMBL3764157) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50140040 (CHEMBL3763905) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139927 (CHEMBL3765741) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50140038 (CHEMBL3764234) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 678 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50140037 (CHEMBL3763452) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO cells assessed as induction of membrane potential change measured every 3 secs for 30 secs by fluoresc... | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||