 Found 107 hits Enz. Inhib. hit(s) with all data for entry = 50028638
Found 107 hits Enz. Inhib. hit(s) with all data for entry = 50028638 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259472

(4-Benzoyl-1-(3-((2-hydroxynaphthalen-1-yl)diazenyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2c(O)ccc3ccccc23)s1 |w:36.39| Show InChI InChI=1S/C35H24N8O5S2/c36-50(47,48)35-41-40-34(49-35)37-33(46)30-28(32(45)23-13-5-2-6-14-23)31(22-11-3-1-4-12-22)43(42-30)25-16-9-15-24(20-25)38-39-29-26-17-8-7-10-21(26)18-19-27(29)44/h1-20,44H,(H2,36,47,48)(H,37,40,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259373

(CHEMBL507085 | tert-Butyl2-((3-(4-benzoyl-5-phenyl...)Show SMILES CC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)OC(C)(C)C |w:4.4| Show InChI InChI=1S/C33H30N8O7S2/c1-19(42)25(30(45)48-33(2,3)4)37-36-22-16-11-17-23(18-22)41-27(20-12-7-5-8-13-20)24(28(43)21-14-9-6-10-15-21)26(40-41)29(44)35-31-38-39-32(49-31)50(34,46)47/h5-18,36H,1-4H3,(H2,34,46,47)(H,35,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259471
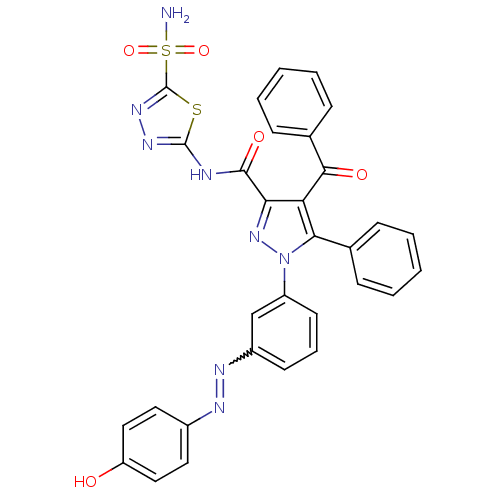
(4-Benzoyl-1-(3-((4-hydroxyphenyl)diazenyl)phenyl)-...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2ccc(O)cc2)s1 |w:36.39| Show InChI InChI=1S/C31H22N8O5S2/c32-46(43,44)31-37-36-30(45-31)33-29(42)26-25(28(41)20-10-5-2-6-11-20)27(19-8-3-1-4-9-19)39(38-26)23-13-7-12-22(18-23)35-34-21-14-16-24(40)17-15-21/h1-18,40H,(H2,32,43,44)(H,33,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259372

(4-Benzoyl-1-(3-((1,3-dioxo-1,3-diphenylpropan-2-yl...)Show SMILES [#7]S(=O)(=O)c1nnc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-c2ccccc2)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6](\[#6](=O)-c3ccccc3)-[#6](=O)-c3ccccc3)c2)s1 Show InChI InChI=1S/C40H28N8O6S2/c41-56(53,54)40-46-45-39(55-40)42-38(52)32-31(35(49)26-16-7-2-8-17-26)34(25-14-5-1-6-15-25)48(47-32)30-23-13-22-29(24-30)43-44-33(36(50)27-18-9-3-10-19-27)37(51)28-20-11-4-12-21-28/h1-24,43H,(H2,41,53,54)(H,42,45,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259371
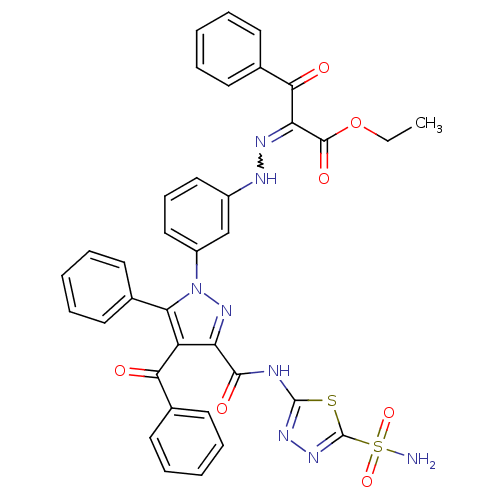
(CHEMBL445982 | Ethyl-2-((3-(4-benzoyl-5-phenyl-3-(...)Show SMILES CCOC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)c1ccccc1 |w:6.6| Show InChI InChI=1S/C36H28N8O7S2/c1-2-51-34(48)29(32(46)24-17-10-5-11-18-24)40-39-25-19-12-20-26(21-25)44-30(22-13-6-3-7-14-22)27(31(45)23-15-8-4-9-16-23)28(43-44)33(47)38-35-41-42-36(52-35)53(37,49)50/h3-21,39H,2H2,1H3,(H2,37,49,50)(H,38,41,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259472

(4-Benzoyl-1-(3-((2-hydroxynaphthalen-1-yl)diazenyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2c(O)ccc3ccccc23)s1 |w:36.39| Show InChI InChI=1S/C35H24N8O5S2/c36-50(47,48)35-41-40-34(49-35)37-33(46)30-28(32(45)23-13-5-2-6-14-23)31(22-11-3-1-4-12-22)43(42-30)25-16-9-15-24(20-25)38-39-29-26-17-8-7-10-21(26)18-19-27(29)44/h1-20,44H,(H2,36,47,48)(H,37,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259369
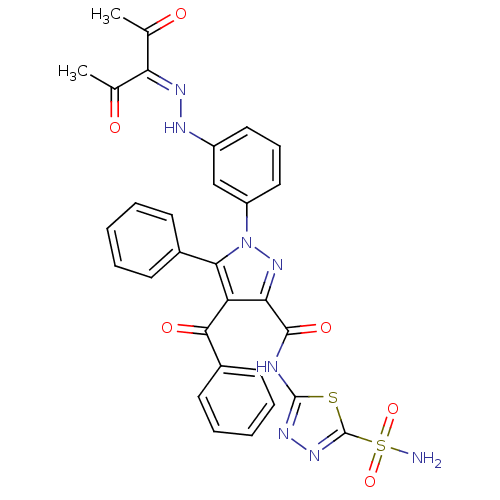
(4-Benzoyl-1-(3-((2,4-dioxopentan-3-yl)diazenyl)phe...)Show SMILES [#6]-[#6](=O)-[#6](=[#7]\[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2nnc(s2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](-[#6])=O Show InChI InChI=1S/C30H24N8O6S2/c1-17(39)24(18(2)40)34-33-21-14-9-15-22(16-21)38-26(19-10-5-3-6-11-19)23(27(41)20-12-7-4-8-13-20)25(37-38)28(42)32-29-35-36-30(45-29)46(31,43)44/h3-16,33H,1-2H3,(H2,31,43,44)(H,32,35,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259375

(4-Benzoyl-1-(3-iodophenyl)-5-phenyl-N-(5-sulfamoyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(I)c2)s1 Show InChI InChI=1S/C25H17IN6O4S2/c26-17-12-7-13-18(14-17)32-21(15-8-3-1-4-9-15)19(22(33)16-10-5-2-6-11-16)20(31-32)23(34)28-24-29-30-25(37-24)38(27,35)36/h1-14H,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259373

(CHEMBL507085 | tert-Butyl2-((3-(4-benzoyl-5-phenyl...)Show SMILES CC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)OC(C)(C)C |w:4.4| Show InChI InChI=1S/C33H30N8O7S2/c1-19(42)25(30(45)48-33(2,3)4)37-36-22-16-11-17-23(18-22)41-27(20-12-7-5-8-13-20)24(28(43)21-14-9-6-10-15-21)26(40-41)29(44)35-31-38-39-32(49-31)50(34,46)47/h5-18,36H,1-4H3,(H2,34,46,47)(H,35,38,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259370
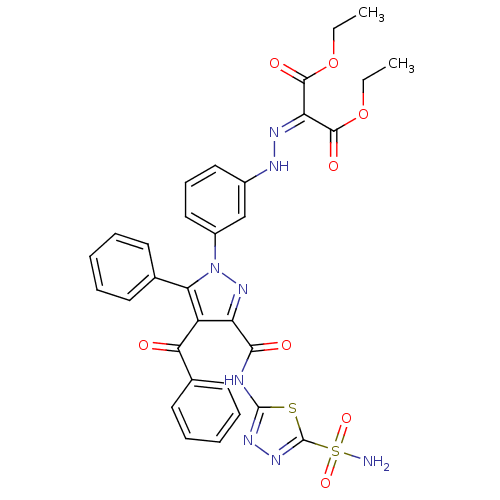
(CHEMBL499578 | Diethyl-2-((3-(4-benzoyl-5-phenyl-3...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#6](=[#7]/[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2nnc(s2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](=O)-[#8]-[#6]-[#6] Show InChI InChI=1S/C32H28N8O8S2/c1-3-47-29(43)25(30(44)48-4-2)36-35-21-16-11-17-22(18-21)40-26(19-12-7-5-8-13-19)23(27(41)20-14-9-6-10-15-20)24(39-40)28(42)34-31-37-38-32(49-31)50(33,45)46/h5-18,35H,3-4H2,1-2H3,(H2,33,45,46)(H,34,37,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259368

(4-Benzoyl-1-(3-(2-(1,3-dioxo-1-phenylbutan-2-ylide...)Show SMILES CC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)c1ccccc1 |w:4.4| Show InChI InChI=1S/C35H26N8O6S2/c1-21(44)28(32(46)24-16-9-4-10-17-24)39-38-25-18-11-19-26(20-25)43-30(22-12-5-2-6-13-22)27(31(45)23-14-7-3-8-15-23)29(42-43)33(47)37-34-40-41-35(50-34)51(36,48)49/h2-20,38H,1H3,(H2,36,48,49)(H,37,40,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259372

(4-Benzoyl-1-(3-((1,3-dioxo-1,3-diphenylpropan-2-yl...)Show SMILES [#7]S(=O)(=O)c1nnc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-c2ccccc2)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6](\[#6](=O)-c3ccccc3)-[#6](=O)-c3ccccc3)c2)s1 Show InChI InChI=1S/C40H28N8O6S2/c41-56(53,54)40-46-45-39(55-40)42-38(52)32-31(35(49)26-16-7-2-8-17-26)34(25-14-5-1-6-15-25)48(47-32)30-23-13-22-29(24-30)43-44-33(36(50)27-18-9-3-10-19-27)37(51)28-20-11-4-12-21-28/h1-24,43H,(H2,41,53,54)(H,42,45,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259473

(4-Benzoyl-1-(2-methyl-1-(2-oxopropyl)-1H-indol-4-y...)Show SMILES CC(=O)Cn1c(C)cc2c(cccc12)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C31H25N7O5S2/c1-18-16-22-23(37(18)17-19(2)39)14-9-15-24(22)38-27(20-10-5-3-6-11-20)25(28(40)21-12-7-4-8-13-21)26(36-38)29(41)33-30-34-35-31(44-30)45(32,42)43/h3-16H,17H2,1-2H3,(H2,32,42,43)(H,33,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259470

(4-Benzoyl-1-(3-hydroxyphenyl)-5-phenyl-N-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(O)c2)s1 Show InChI InChI=1S/C25H18N6O5S2/c26-38(35,36)25-29-28-24(37-25)27-23(34)20-19(22(33)16-10-5-2-6-11-16)21(15-8-3-1-4-9-15)31(30-20)17-12-7-13-18(32)14-17/h1-14,32H,(H2,26,35,36)(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259473

(4-Benzoyl-1-(2-methyl-1-(2-oxopropyl)-1H-indol-4-y...)Show SMILES CC(=O)Cn1c(C)cc2c(cccc12)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C31H25N7O5S2/c1-18-16-22-23(37(18)17-19(2)39)14-9-15-24(22)38-27(20-10-5-3-6-11-20)25(28(40)21-12-7-4-8-13-21)26(36-38)29(41)33-30-34-35-31(44-30)45(32,42)43/h3-16H,17H2,1-2H3,(H2,32,42,43)(H,33,34,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259369

(4-Benzoyl-1-(3-((2,4-dioxopentan-3-yl)diazenyl)phe...)Show SMILES [#6]-[#6](=O)-[#6](=[#7]\[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2nnc(s2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](-[#6])=O Show InChI InChI=1S/C30H24N8O6S2/c1-17(39)24(18(2)40)34-33-21-14-9-15-22(16-21)38-26(19-10-5-3-6-11-19)23(27(41)20-12-7-4-8-13-20)25(37-38)28(42)32-29-35-36-30(45-29)46(31,43)44/h3-16,33H,1-2H3,(H2,31,43,44)(H,32,35,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259368

(4-Benzoyl-1-(3-(2-(1,3-dioxo-1-phenylbutan-2-ylide...)Show SMILES CC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)c1ccccc1 |w:4.4| Show InChI InChI=1S/C35H26N8O6S2/c1-21(44)28(32(46)24-16-9-4-10-17-24)39-38-25-18-11-19-26(20-25)43-30(22-12-5-2-6-13-22)27(31(45)23-14-7-3-8-15-23)29(42-43)33(47)37-34-40-41-35(50-34)51(36,48)49/h2-20,38H,1H3,(H2,36,48,49)(H,37,40,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259374
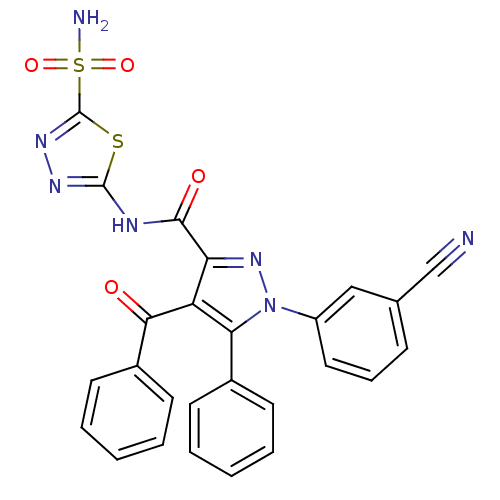
(4-Benzoyl-1-(3-cyanophenyl)-5-phenyl-N-(5-sulfamoy...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)C#N)s1 Show InChI InChI=1S/C26H17N7O4S2/c27-15-16-8-7-13-19(14-16)33-22(17-9-3-1-4-10-17)20(23(34)18-11-5-2-6-12-18)21(32-33)24(35)29-25-30-31-26(38-25)39(28,36)37/h1-14H,(H2,28,36,37)(H,29,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259323

(1-(3-Aminophenyl)-4-benzoyl-5-phenyl-N-(5-sulfamoy...)Show SMILES Nc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C25H19N7O4S2/c26-17-12-7-13-18(14-17)32-21(15-8-3-1-4-9-15)19(22(33)16-10-5-2-6-11-16)20(31-32)23(34)28-24-29-30-25(37-24)38(27,35)36/h1-14H,26H2,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259470

(4-Benzoyl-1-(3-hydroxyphenyl)-5-phenyl-N-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(O)c2)s1 Show InChI InChI=1S/C25H18N6O5S2/c26-38(35,36)25-29-28-24(37-25)27-23(34)20-19(22(33)16-10-5-2-6-11-16)21(15-8-3-1-4-9-15)31(30-20)17-12-7-13-18(32)14-17/h1-14,32H,(H2,26,35,36)(H,27,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259374

(4-Benzoyl-1-(3-cyanophenyl)-5-phenyl-N-(5-sulfamoy...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)C#N)s1 Show InChI InChI=1S/C26H17N7O4S2/c27-15-16-8-7-13-19(14-16)33-22(17-9-3-1-4-10-17)20(23(34)18-11-5-2-6-12-18)21(32-33)24(35)29-25-30-31-26(38-25)39(28,36)37/h1-14H,(H2,28,36,37)(H,29,30,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259471

(4-Benzoyl-1-(3-((4-hydroxyphenyl)diazenyl)phenyl)-...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2ccc(O)cc2)s1 |w:36.39| Show InChI InChI=1S/C31H22N8O5S2/c32-46(43,44)31-37-36-30(45-31)33-29(42)26-25(28(41)20-10-5-2-6-11-20)27(19-8-3-1-4-9-19)39(38-26)23-13-7-12-22(18-23)35-34-21-14-16-24(40)17-15-21/h1-18,40H,(H2,32,43,44)(H,33,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259371

(CHEMBL445982 | Ethyl-2-((3-(4-benzoyl-5-phenyl-3-(...)Show SMILES CCOC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)c1ccccc1 |w:6.6| Show InChI InChI=1S/C36H28N8O7S2/c1-2-51-34(48)29(32(46)24-17-10-5-11-18-24)40-39-25-19-12-20-26(21-25)44-30(22-13-6-3-7-14-22)27(31(45)23-15-8-4-9-16-23)28(43-44)33(47)38-35-41-42-36(52-35)53(37,49)50/h3-21,39H,2H2,1H3,(H2,37,49,50)(H,38,41,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259370

(CHEMBL499578 | Diethyl-2-((3-(4-benzoyl-5-phenyl-3...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#6](=[#7]/[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2nnc(s2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](=O)-[#8]-[#6]-[#6] Show InChI InChI=1S/C32H28N8O8S2/c1-3-47-29(43)25(30(44)48-4-2)36-35-21-16-11-17-22(18-21)40-26(19-12-7-5-8-13-19)23(27(41)20-14-9-6-10-15-20)24(39-40)28(42)34-31-37-38-32(49-31)50(33,45)46/h5-18,35H,3-4H2,1-2H3,(H2,33,45,46)(H,34,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259367
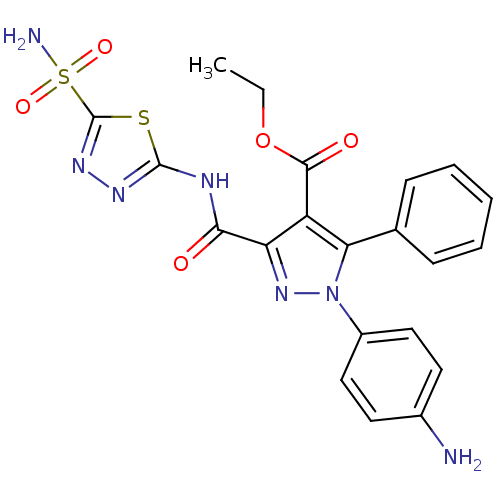
(CHEMBL513122 | Ethyl-1-(4-aminophenyl)-5-phenyl-3-...)Show SMILES CCOC(=O)c1c(nn(c1-c1ccccc1)-c1ccc(N)cc1)C(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C21H19N7O5S2/c1-2-33-19(30)15-16(18(29)24-20-25-26-21(34-20)35(23,31)32)27-28(14-10-8-13(22)9-11-14)17(15)12-6-4-3-5-7-12/h3-11H,2,22H2,1H3,(H2,23,31,32)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259324
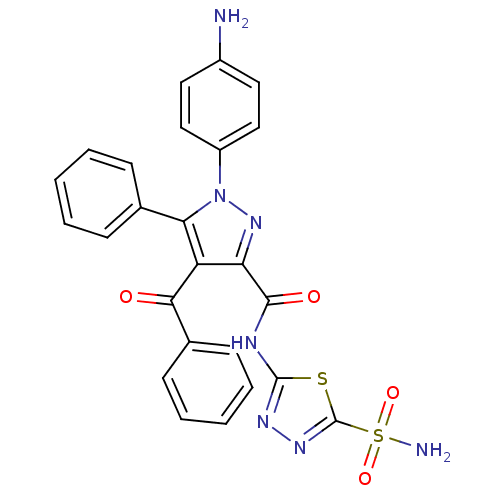
(1-(4-Aminophenyl)-4-benzoyl-5-phenyl-N-(5-sulfamoy...)Show SMILES Nc1ccc(cc1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C25H19N7O4S2/c26-17-11-13-18(14-12-17)32-21(15-7-3-1-4-8-15)19(22(33)16-9-5-2-6-10-16)20(31-32)23(34)28-24-29-30-25(37-24)38(27,35)36/h1-14H,26H2,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259375

(4-Benzoyl-1-(3-iodophenyl)-5-phenyl-N-(5-sulfamoyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(I)c2)s1 Show InChI InChI=1S/C25H17IN6O4S2/c26-17-12-7-13-18(14-17)32-21(15-8-3-1-4-9-15)19(22(33)16-10-5-2-6-11-16)20(31-32)23(34)28-24-29-30-25(37-24)38(27,35)36/h1-14H,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259324

(1-(4-Aminophenyl)-4-benzoyl-5-phenyl-N-(5-sulfamoy...)Show SMILES Nc1ccc(cc1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C25H19N7O4S2/c26-17-11-13-18(14-12-17)32-21(15-7-3-1-4-8-15)19(22(33)16-9-5-2-6-10-16)20(31-32)23(34)28-24-29-30-25(37-24)38(27,35)36/h1-14H,26H2,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259323

(1-(3-Aminophenyl)-4-benzoyl-5-phenyl-N-(5-sulfamoy...)Show SMILES Nc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C25H19N7O4S2/c26-17-12-7-13-18(14-17)32-21(15-8-3-1-4-9-15)19(22(33)16-10-5-2-6-11-16)20(31-32)23(34)28-24-29-30-25(37-24)38(27,35)36/h1-14H,26H2,(H2,27,35,36)(H,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259367

(CHEMBL513122 | Ethyl-1-(4-aminophenyl)-5-phenyl-3-...)Show SMILES CCOC(=O)c1c(nn(c1-c1ccccc1)-c1ccc(N)cc1)C(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C21H19N7O5S2/c1-2-33-19(30)15-16(18(29)24-20-25-26-21(34-20)35(23,31)32)27-28(14-10-8-13(22)9-11-14)17(15)12-6-4-3-5-7-12/h3-11H,2,22H2,1H3,(H2,23,31,32)(H,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259325
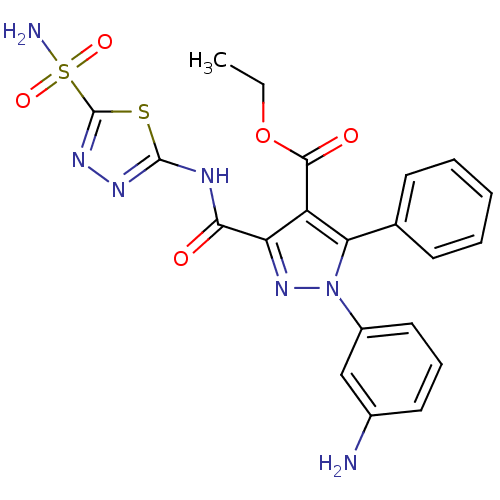
(CHEMBL467098 | Ethyl-1-(3-aminophenyl)-5-phenyl-3-...)Show SMILES CCOC(=O)c1c(nn(c1-c1ccccc1)-c1cccc(N)c1)C(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C21H19N7O5S2/c1-2-33-19(30)15-16(18(29)24-20-25-26-21(34-20)35(23,31)32)27-28(14-10-6-9-13(22)11-14)17(15)12-7-4-3-5-8-12/h3-11H,2,22H2,1H3,(H2,23,31,32)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10868
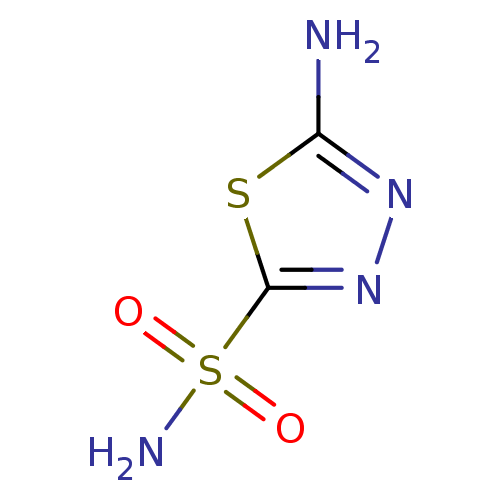
(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10868

(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by Lineweaver-Burk plot |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259472

(4-Benzoyl-1-(3-((2-hydroxynaphthalen-1-yl)diazenyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2c(O)ccc3ccccc23)s1 |w:36.39| Show InChI InChI=1S/C35H24N8O5S2/c36-50(47,48)35-41-40-34(49-35)37-33(46)30-28(32(45)23-13-5-2-6-14-23)31(22-11-3-1-4-12-22)43(42-30)25-16-9-15-24(20-25)38-39-29-26-17-8-7-10-21(26)18-19-27(29)44/h1-20,44H,(H2,36,47,48)(H,37,40,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259470

(4-Benzoyl-1-(3-hydroxyphenyl)-5-phenyl-N-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(O)c2)s1 Show InChI InChI=1S/C25H18N6O5S2/c26-38(35,36)25-29-28-24(37-25)27-23(34)20-19(22(33)16-10-5-2-6-11-16)21(15-8-3-1-4-9-15)31(30-20)17-12-7-13-18(32)14-17/h1-14,32H,(H2,26,35,36)(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259325

(CHEMBL467098 | Ethyl-1-(3-aminophenyl)-5-phenyl-3-...)Show SMILES CCOC(=O)c1c(nn(c1-c1ccccc1)-c1cccc(N)c1)C(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C21H19N7O5S2/c1-2-33-19(30)15-16(18(29)24-20-25-26-21(34-20)35(23,31)32)27-28(14-10-6-9-13(22)11-14)17(15)12-7-4-3-5-8-12/h3-11H,2,22H2,1H3,(H2,23,31,32)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259367

(CHEMBL513122 | Ethyl-1-(4-aminophenyl)-5-phenyl-3-...)Show SMILES CCOC(=O)c1c(nn(c1-c1ccccc1)-c1ccc(N)cc1)C(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C21H19N7O5S2/c1-2-33-19(30)15-16(18(29)24-20-25-26-21(34-20)35(23,31)32)27-28(14-10-8-13(22)9-11-14)17(15)12-6-4-3-5-7-12/h3-11H,2,22H2,1H3,(H2,23,31,32)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259472

(4-Benzoyl-1-(3-((2-hydroxynaphthalen-1-yl)diazenyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2c(O)ccc3ccccc23)s1 |w:36.39| Show InChI InChI=1S/C35H24N8O5S2/c36-50(47,48)35-41-40-34(49-35)37-33(46)30-28(32(45)23-13-5-2-6-14-23)31(22-11-3-1-4-12-22)43(42-30)25-16-9-15-24(20-25)38-39-29-26-17-8-7-10-21(26)18-19-27(29)44/h1-20,44H,(H2,36,47,48)(H,37,40,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259372

(4-Benzoyl-1-(3-((1,3-dioxo-1,3-diphenylpropan-2-yl...)Show SMILES [#7]S(=O)(=O)c1nnc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-c2ccccc2)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6](\[#6](=O)-c3ccccc3)-[#6](=O)-c3ccccc3)c2)s1 Show InChI InChI=1S/C40H28N8O6S2/c41-56(53,54)40-46-45-39(55-40)42-38(52)32-31(35(49)26-16-7-2-8-17-26)34(25-14-5-1-6-15-25)48(47-32)30-23-13-22-29(24-30)43-44-33(36(50)27-18-9-3-10-19-27)37(51)28-20-11-4-12-21-28/h1-24,43H,(H2,41,53,54)(H,42,45,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259373

(CHEMBL507085 | tert-Butyl2-((3-(4-benzoyl-5-phenyl...)Show SMILES CC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)OC(C)(C)C |w:4.4| Show InChI InChI=1S/C33H30N8O7S2/c1-19(42)25(30(45)48-33(2,3)4)37-36-22-16-11-17-23(18-22)41-27(20-12-7-5-8-13-20)24(28(43)21-14-9-6-10-15-21)26(40-41)29(44)35-31-38-39-32(49-31)50(34,46)47/h5-18,36H,1-4H3,(H2,34,46,47)(H,35,38,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 1 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259471

(4-Benzoyl-1-(3-((4-hydroxyphenyl)diazenyl)phenyl)-...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2ccc(O)cc2)s1 |w:36.39| Show InChI InChI=1S/C31H22N8O5S2/c32-46(43,44)31-37-36-30(45-31)33-29(42)26-25(28(41)20-10-5-2-6-11-20)27(19-8-3-1-4-9-19)39(38-26)23-13-7-12-22(18-23)35-34-21-14-16-24(40)17-15-21/h1-18,40H,(H2,32,43,44)(H,33,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259374

(4-Benzoyl-1-(3-cyanophenyl)-5-phenyl-N-(5-sulfamoy...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)C#N)s1 Show InChI InChI=1S/C26H17N7O4S2/c27-15-16-8-7-13-19(14-16)33-22(17-9-3-1-4-10-17)20(23(34)18-11-5-2-6-12-18)21(32-33)24(35)29-25-30-31-26(38-25)39(28,36)37/h1-14H,(H2,28,36,37)(H,29,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259371

(CHEMBL445982 | Ethyl-2-((3-(4-benzoyl-5-phenyl-3-(...)Show SMILES CCOC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)c1ccccc1 |w:6.6| Show InChI InChI=1S/C36H28N8O7S2/c1-2-51-34(48)29(32(46)24-17-10-5-11-18-24)40-39-25-19-12-20-26(21-25)44-30(22-13-6-3-7-14-22)27(31(45)23-15-8-4-9-16-23)28(43-44)33(47)38-35-41-42-36(52-35)53(37,49)50/h3-21,39H,2H2,1H3,(H2,37,49,50)(H,38,41,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259471

(4-Benzoyl-1-(3-((4-hydroxyphenyl)diazenyl)phenyl)-...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=Nc2ccc(O)cc2)s1 |w:36.39| Show InChI InChI=1S/C31H22N8O5S2/c32-46(43,44)31-37-36-30(45-31)33-29(42)26-25(28(41)20-10-5-2-6-11-20)27(19-8-3-1-4-9-19)39(38-26)23-13-7-12-22(18-23)35-34-21-14-16-24(40)17-15-21/h1-18,40H,(H2,32,43,44)(H,33,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259325

(CHEMBL467098 | Ethyl-1-(3-aminophenyl)-5-phenyl-3-...)Show SMILES CCOC(=O)c1c(nn(c1-c1ccccc1)-c1cccc(N)c1)C(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C21H19N7O5S2/c1-2-33-19(30)15-16(18(29)24-20-25-26-21(34-20)35(23,31)32)27-28(14-10-6-9-13(22)11-14)17(15)12-7-4-3-5-8-12/h3-11H,2,22H2,1H3,(H2,23,31,32)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259370

(CHEMBL499578 | Diethyl-2-((3-(4-benzoyl-5-phenyl-3...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#6](=[#7]/[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2nnc(s2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](=O)-[#8]-[#6]-[#6] Show InChI InChI=1S/C32H28N8O8S2/c1-3-47-29(43)25(30(44)48-4-2)36-35-21-16-11-17-22(18-21)40-26(19-12-7-5-8-13-19)23(27(41)20-14-9-6-10-15-20)24(39-40)28(42)34-31-37-38-32(49-31)50(33,45)46/h5-18,35H,3-4H2,1-2H3,(H2,33,45,46)(H,34,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of CO2-hydratase activity of human carbonic anhydrase 1 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50259373

(CHEMBL507085 | tert-Butyl2-((3-(4-benzoyl-5-phenyl...)Show SMILES CC(=O)C(=NNc1cccc(c1)-n1nc(C(=O)Nc2nnc(s2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)C(=O)OC(C)(C)C |w:4.4| Show InChI InChI=1S/C33H30N8O7S2/c1-19(42)25(30(45)48-33(2,3)4)37-36-22-16-11-17-23(18-22)41-27(20-12-7-5-8-13-20)24(28(43)21-14-9-6-10-15-21)26(40-41)29(44)35-31-38-39-32(49-31)50(34,46)47/h5-18,36H,1-4H3,(H2,34,46,47)(H,35,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 2 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50259372

(4-Benzoyl-1-(3-((1,3-dioxo-1,3-diphenylpropan-2-yl...)Show SMILES [#7]S(=O)(=O)c1nnc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-c2ccccc2)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6](\[#6](=O)-c3ccccc3)-[#6](=O)-c3ccccc3)c2)s1 Show InChI InChI=1S/C40H28N8O6S2/c41-56(53,54)40-46-45-39(55-40)42-38(52)32-31(35(49)26-16-7-2-8-17-26)34(25-14-5-1-6-15-25)48(47-32)30-23-13-22-29(24-30)43-44-33(36(50)27-18-9-3-10-19-27)37(51)28-20-11-4-12-21-28/h1-24,43H,(H2,41,53,54)(H,42,45,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of human carbonic anhydrase 1 by CO2 hydration method |
Bioorg Med Chem 17: 3295-301 (2009)
Article DOI: 10.1016/j.bmc.2009.03.048
BindingDB Entry DOI: 10.7270/Q2DZ087F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data