

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145168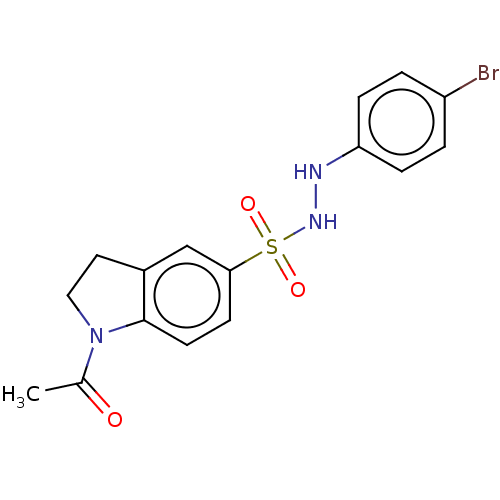 (CHEMBL3764319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145157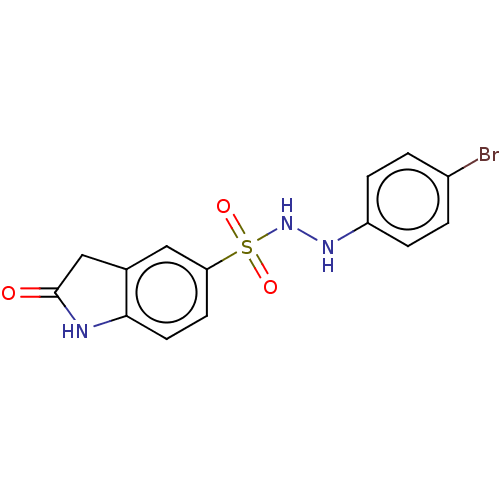 (CHEMBL3763166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145166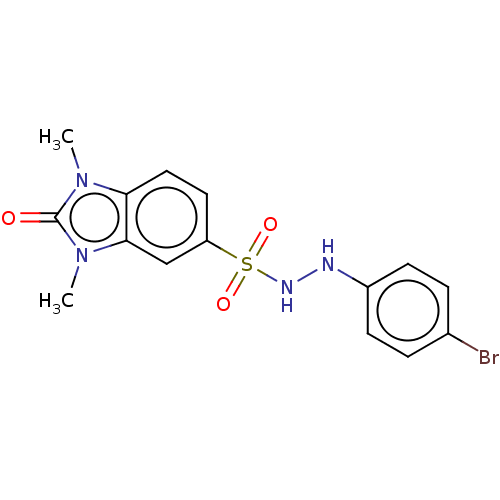 (CHEMBL3763493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145159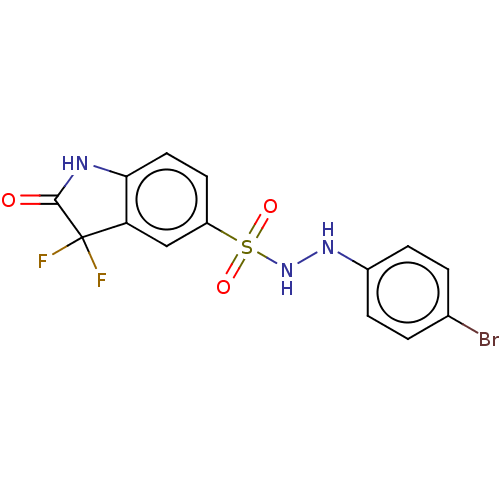 (CHEMBL3765205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145164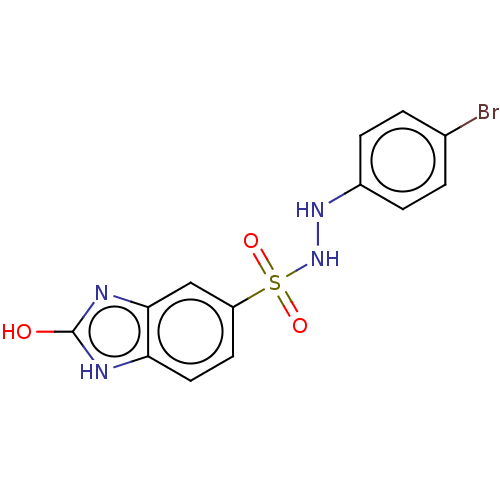 (CHEMBL3765698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145169 (CHEMBL3763974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145160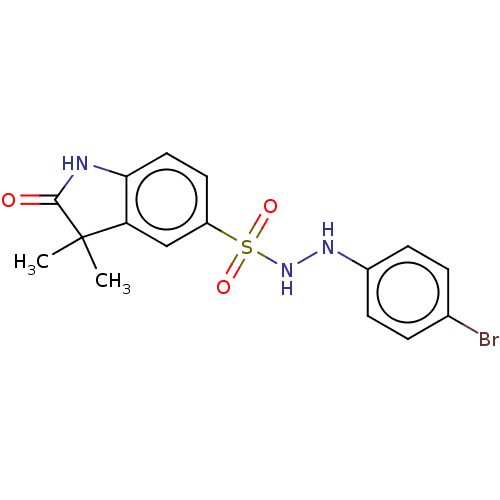 (CHEMBL3764846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145161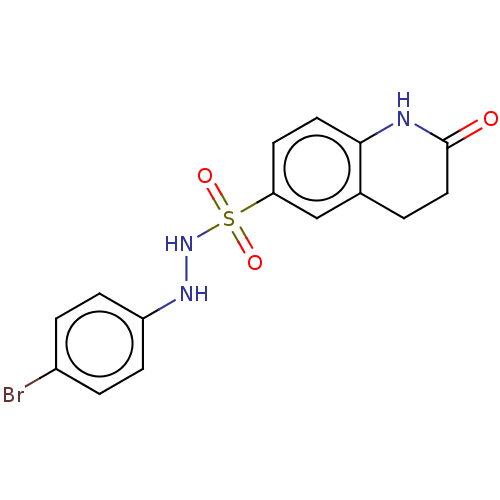 (CHEMBL3765545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145158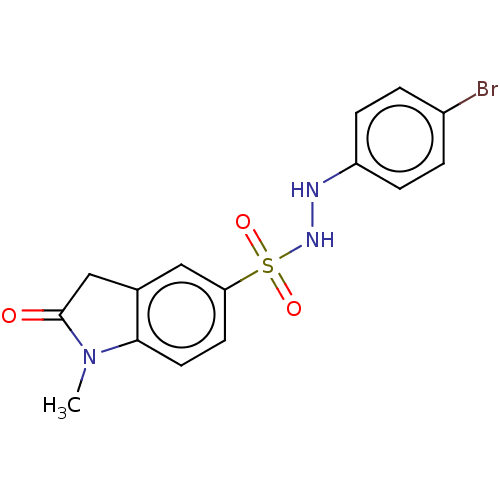 (CHEMBL3764144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145170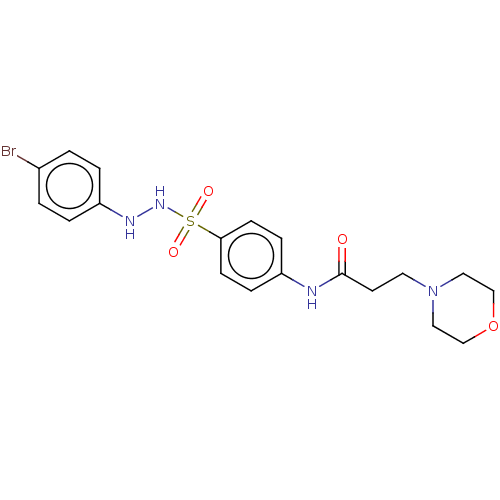 (CHEMBL3765405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145162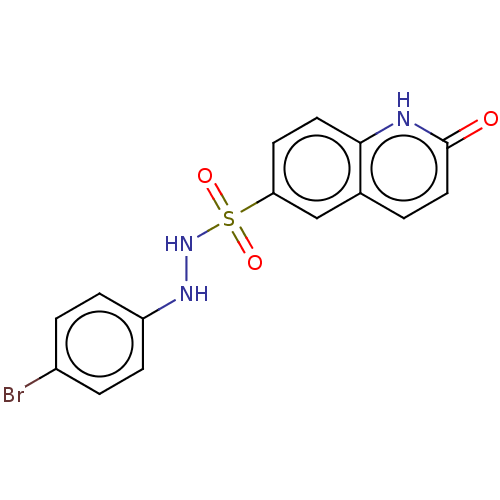 (CHEMBL3764508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145163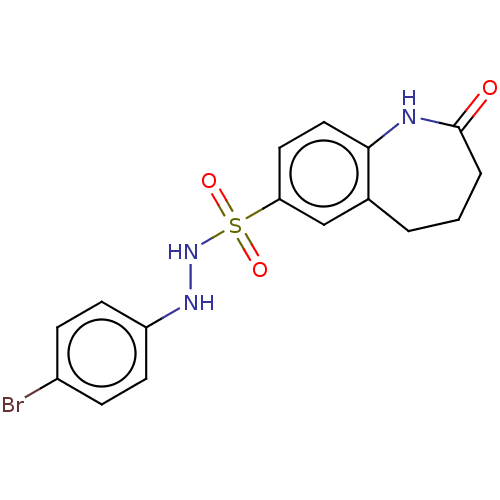 (CHEMBL3763962) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145165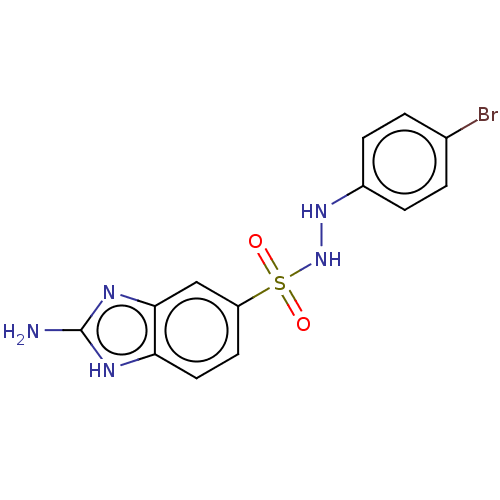 (CHEMBL3765220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145167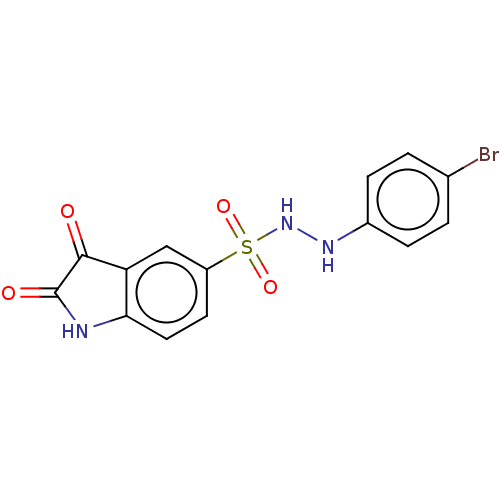 (CHEMBL3764028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145169 (CHEMBL3763974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145168 (CHEMBL3764319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 231 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145157 (CHEMBL3763166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145166 (CHEMBL3763493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145165 (CHEMBL3765220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145164 (CHEMBL3765698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145163 (CHEMBL3763962) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145162 (CHEMBL3764508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 212 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145161 (CHEMBL3765545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145160 (CHEMBL3764846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145159 (CHEMBL3765205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145170 (CHEMBL3765405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 217 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145158 (CHEMBL3764144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 365 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||