 Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50047100
Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50047100 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50108620
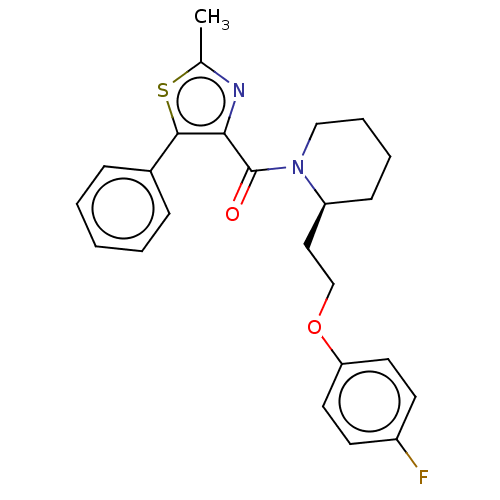
(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50148575
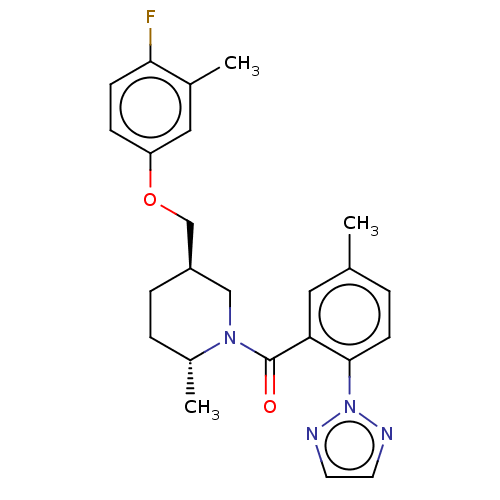
(CHEMBL3770503)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104692
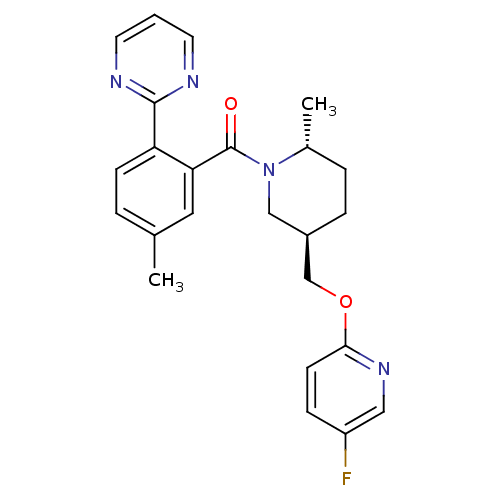
(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148575

(CHEMBL3770503)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50108620

(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104692

(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50148572
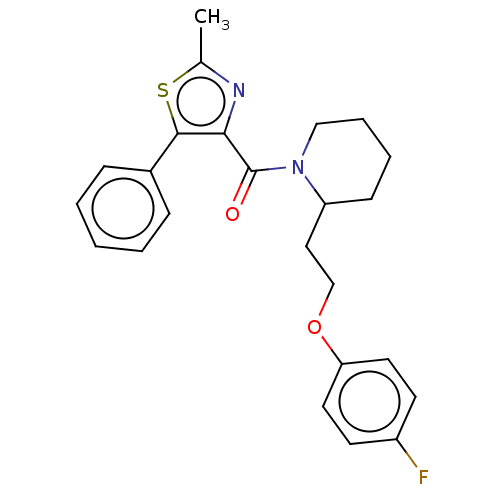
(CHEMBL3771050)Show SMILES Cc1nc(C(=O)N2CCCCC2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148572

(CHEMBL3771050)Show SMILES Cc1nc(C(=O)N2CCCCC2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060944
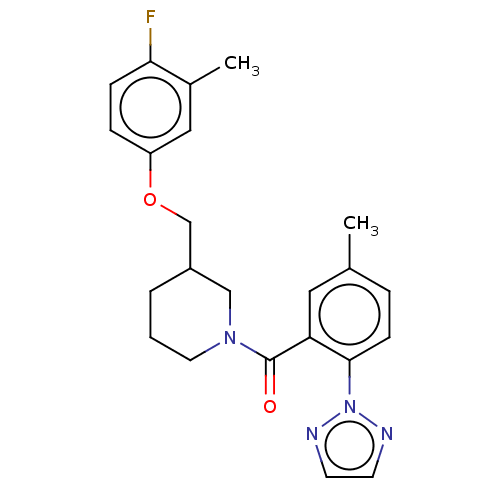
(CHEMBL3394825)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(COc2ccc(F)c(C)c2)C1)-n1nccn1 Show InChI InChI=1S/C23H25FN4O2/c1-16-5-8-22(28-25-9-10-26-28)20(12-16)23(29)27-11-3-4-18(14-27)15-30-19-6-7-21(24)17(2)13-19/h5-10,12-13,18H,3-4,11,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060944

(CHEMBL3394825)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(COc2ccc(F)c(C)c2)C1)-n1nccn1 Show InChI InChI=1S/C23H25FN4O2/c1-16-5-8-22(28-25-9-10-26-28)20(12-16)23(29)27-11-3-4-18(14-27)15-30-19-6-7-21(24)17(2)13-19/h5-10,12-13,18H,3-4,11,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50148574

(CHEMBL3769978)Show SMILES C[C@@H]1CC[C@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50108619

(CHEMBL3597954)Show SMILES Cc1nc(C(=O)N2CCC[C@@H](COc3ccc(F)cc3)C2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C23H23FN2O2S/c1-16-25-21(22(29-16)18-7-3-2-4-8-18)23(27)26-13-5-6-17(14-26)15-28-20-11-9-19(24)10-12-20/h2-4,7-12,17H,5-6,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148574

(CHEMBL3769978)Show SMILES C[C@@H]1CC[C@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50108619

(CHEMBL3597954)Show SMILES Cc1nc(C(=O)N2CCC[C@@H](COc3ccc(F)cc3)C2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C23H23FN2O2S/c1-16-25-21(22(29-16)18-7-3-2-4-8-18)23(27)26-13-5-6-17(14-26)15-28-20-11-9-19(24)10-12-20/h2-4,7-12,17H,5-6,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM154927
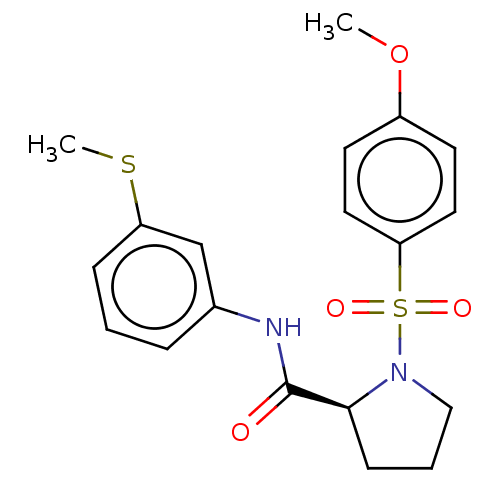
(US9000029, 6)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCC[C@H]1C(=O)Nc1cccc(SC)c1 Show InChI InChI=1S/C19H22N2O4S2/c1-25-15-8-10-17(11-9-15)27(23,24)21-12-4-7-18(21)19(22)20-14-5-3-6-16(13-14)26-2/h3,5-6,8-11,13,18H,4,7,12H2,1-2H3,(H,20,22)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50292929
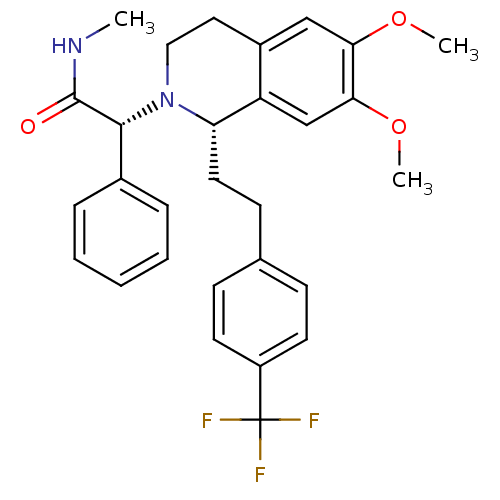
(CHEMBL455136 | almorexant)Show SMILES CNC(=O)[C@H](N1CCc2cc(OC)c(OC)cc2[C@@H]1CCc1ccc(cc1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C29H31F3N2O3/c1-33-28(35)27(20-7-5-4-6-8-20)34-16-15-21-17-25(36-2)26(37-3)18-23(21)24(34)14-11-19-9-12-22(13-10-19)29(30,31)32/h4-10,12-13,17-18,24,27H,11,14-16H2,1-3H3,(H,33,35)/t24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM154922
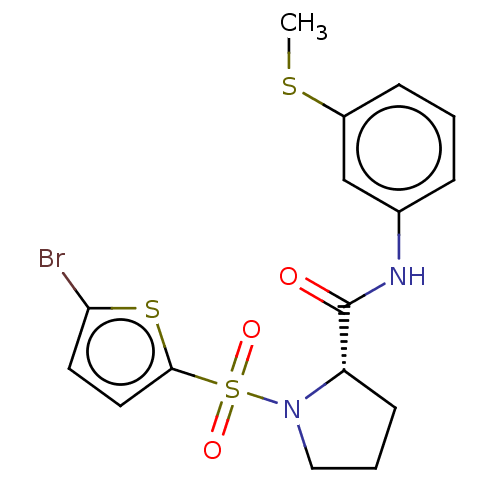
(US9000029, 1)Show SMILES CSc1cccc(NC(=O)[C@@H]2CCCN2S(=O)(=O)c2ccc(Br)s2)c1 Show InChI InChI=1S/C16H17BrN2O3S3/c1-23-12-5-2-4-11(10-12)18-16(20)13-6-3-9-19(13)25(21,22)15-8-7-14(17)24-15/h2,4-5,7-8,10,13H,3,6,9H2,1H3,(H,18,20)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104692

(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHO cell membranes assessed as inhibition of orexin-A-induced intracellular calcium release after 120 ... |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM154947

(US9000029, 26)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCC[C@H]1C(=O)Nc1cc(C)cc(C)c1 Show InChI InChI=1S/C20H24N2O4S/c1-14-11-15(2)13-16(12-14)21-20(23)19-5-4-10-22(19)27(24,25)18-8-6-17(26-3)7-9-18/h6-9,11-13,19H,4-5,10H2,1-3H3,(H,21,23)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104692

(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R expressed in CHO cell membranes assessed as inhibition of orexin-A-induced intracellular calcium release after 120 ... |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50108620

(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148571
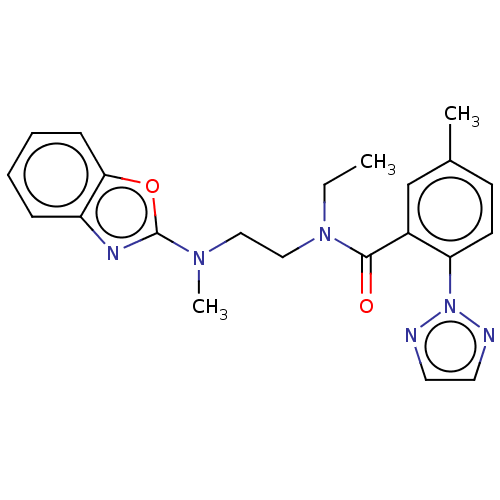
(CHEMBL3770745)Show SMILES CCN(CCN(C)c1nc2ccccc2o1)C(=O)c1cc(C)ccc1-n1nccn1 Show InChI InChI=1S/C22H24N6O2/c1-4-27(14-13-26(3)22-25-18-7-5-6-8-20(18)30-22)21(29)17-15-16(2)9-10-19(17)28-23-11-12-24-28/h5-12,15H,4,13-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50292929

(CHEMBL455136 | almorexant)Show SMILES CNC(=O)[C@H](N1CCc2cc(OC)c(OC)cc2[C@@H]1CCc1ccc(cc1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C29H31F3N2O3/c1-33-28(35)27(20-7-5-4-6-8-20)34-16-15-21-17-25(36-2)26(37-3)18-23(21)24(34)14-11-19-9-12-22(13-10-19)29(30,31)32/h4-10,12-13,17-18,24,27H,11,14-16H2,1-3H3,(H,33,35)/t24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50108620

(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148576
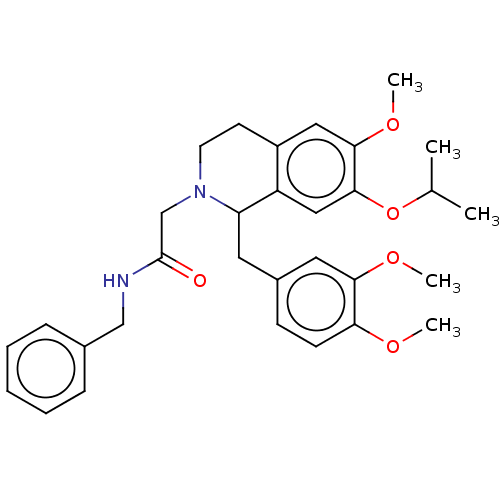
(CHEMBL3771066)Show SMILES COc1ccc(CC2N(CC(=O)NCc3ccccc3)CCc3cc(OC)c(OC(C)C)cc23)cc1OC Show InChI InChI=1S/C31H38N2O5/c1-21(2)38-30-18-25-24(17-29(30)37-5)13-14-33(20-31(34)32-19-22-9-7-6-8-10-22)26(25)15-23-11-12-27(35-3)28(16-23)36-4/h6-12,16-18,21,26H,13-15,19-20H2,1-5H3,(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50148571

(CHEMBL3770745)Show SMILES CCN(CCN(C)c1nc2ccccc2o1)C(=O)c1cc(C)ccc1-n1nccn1 Show InChI InChI=1S/C22H24N6O2/c1-4-27(14-13-26(3)22-25-18-7-5-6-8-20(18)30-22)21(29)17-15-16(2)9-10-19(17)28-23-11-12-24-28/h5-12,15H,4,13-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028059
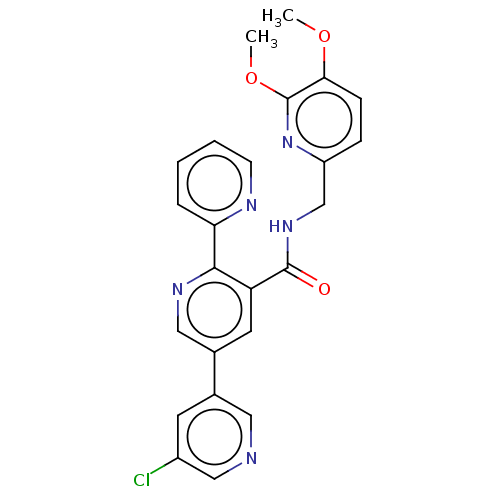
(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R expressed in CHO cells by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50292952

((+/-)-2-(1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-3,4...)Show SMILES COc1ccc2CCC(NC(=O)CN3CCc4cc(OC)c(OC)cc4C3Cc3ccc(OC)c(OC)c3)c2c1 Show InChI InChI=1S/C32H38N2O6/c1-36-23-9-7-21-8-10-26(24(21)17-23)33-32(35)19-34-13-12-22-16-30(39-4)31(40-5)18-25(22)27(34)14-20-6-11-28(37-2)29(15-20)38-3/h6-7,9,11,15-18,26-27H,8,10,12-14,19H2,1-5H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM154922

(US9000029, 1)Show SMILES CSc1cccc(NC(=O)[C@@H]2CCCN2S(=O)(=O)c2ccc(Br)s2)c1 Show InChI InChI=1S/C16H17BrN2O3S3/c1-23-12-5-2-4-11(10-12)18-16(20)13-6-3-9-19(13)25(21,22)15-8-7-14(17)24-15/h2,4-5,7-8,10,13H,3,6,9H2,1H3,(H,18,20)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318697
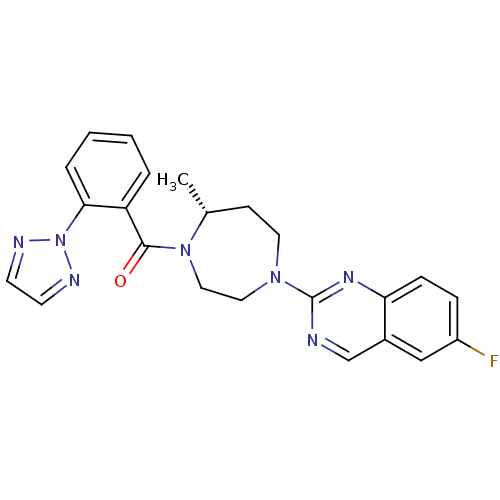
(6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C23H22FN7O/c1-16-8-11-29(23-25-15-17-14-18(24)6-7-20(17)28-23)12-13-30(16)22(32)19-4-2-3-5-21(19)31-26-9-10-27-31/h2-7,9-10,14-16H,8,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50258741
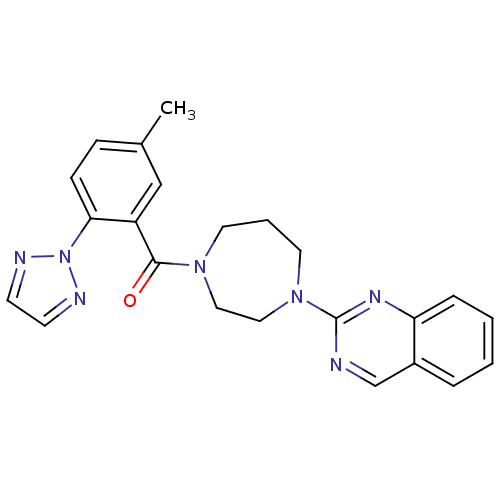
((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCN(CC1)c1ncc2ccccc2n1)-n1nccn1 Show InChI InChI=1S/C23H23N7O/c1-17-7-8-21(30-25-9-10-26-30)19(15-17)22(31)28-11-4-12-29(14-13-28)23-24-16-18-5-2-3-6-20(18)27-23/h2-3,5-10,15-16H,4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318697

(6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C23H22FN7O/c1-16-8-11-29(23-25-15-17-14-18(24)6-7-20(17)28-23)12-13-30(16)22(32)19-4-2-3-5-21(19)31-26-9-10-27-31/h2-7,9-10,14-16H,8,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50258741

((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCN(CC1)c1ncc2ccccc2n1)-n1nccn1 Show InChI InChI=1S/C23H23N7O/c1-17-7-8-21(30-25-9-10-26-30)19(15-17)22(31)28-11-4-12-29(14-13-28)23-24-16-18-5-2-3-6-20(18)27-23/h2-3,5-10,15-16H,4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM154927

(US9000029, 6)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCC[C@H]1C(=O)Nc1cccc(SC)c1 Show InChI InChI=1S/C19H22N2O4S2/c1-25-15-8-10-17(11-9-15)27(23,24)21-12-4-7-18(21)19(22)20-14-5-3-6-16(13-14)26-2/h3,5-6,8-11,13,18H,4,7,12H2,1-2H3,(H,20,22)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50292933
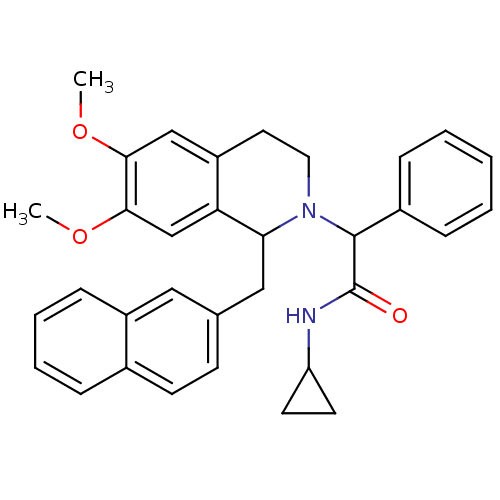
(CHEMBL490082 | N-cyclopropyl-2-(6,7-dimethoxy-1-(n...)Show SMILES COc1cc2CCN(C(C(=O)NC3CC3)c3ccccc3)C(Cc3ccc4ccccc4c3)c2cc1OC Show InChI InChI=1S/C33H34N2O3/c1-37-30-20-26-16-17-35(32(24-9-4-3-5-10-24)33(36)34-27-14-15-27)29(28(26)21-31(30)38-2)19-22-12-13-23-8-6-7-11-25(23)18-22/h3-13,18,20-21,27,29,32H,14-17,19H2,1-2H3,(H,34,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM154947

(US9000029, 26)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCC[C@H]1C(=O)Nc1cc(C)cc(C)c1 Show InChI InChI=1S/C20H24N2O4S/c1-14-11-15(2)13-16(12-14)21-20(23)19-5-4-10-22(19)27(24,25)18-8-6-17(26-3)7-9-18/h6-9,11-13,19H,4-5,10H2,1-3H3,(H,21,23)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium release by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50292933

(CHEMBL490082 | N-cyclopropyl-2-(6,7-dimethoxy-1-(n...)Show SMILES COc1cc2CCN(C(C(=O)NC3CC3)c3ccccc3)C(Cc3ccc4ccccc4c3)c2cc1OC Show InChI InChI=1S/C33H34N2O3/c1-37-30-20-26-16-17-35(32(24-9-4-3-5-10-24)33(36)34-27-14-15-27)29(28(26)21-31(30)38-2)19-22-12-13-23-8-6-7-11-25(23)18-22/h3-13,18,20-21,27,29,32H,14-17,19H2,1-2H3,(H,34,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092810
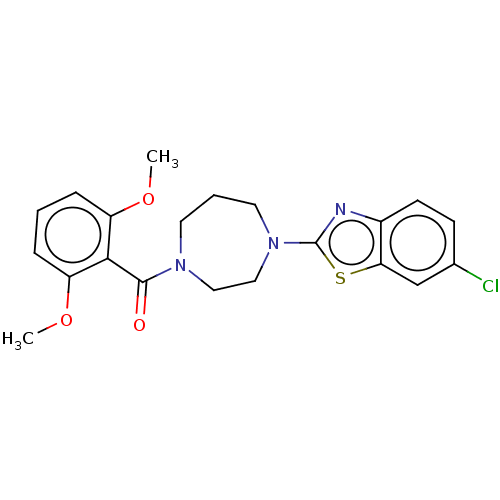
(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50292952

((+/-)-2-(1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-3,4...)Show SMILES COc1ccc2CCC(NC(=O)CN3CCc4cc(OC)c(OC)cc4C3Cc3ccc(OC)c(OC)c3)c2c1 Show InChI InChI=1S/C32H38N2O6/c1-36-23-9-7-21-8-10-26(24(21)17-23)33-32(35)19-34-13-12-22-16-30(39-4)31(40-5)18-25(22)27(34)14-20-6-11-28(37-2)29(15-20)38-3/h6-7,9,11,15-18,26-27H,8,10,12-14,19H2,1-5H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50292939
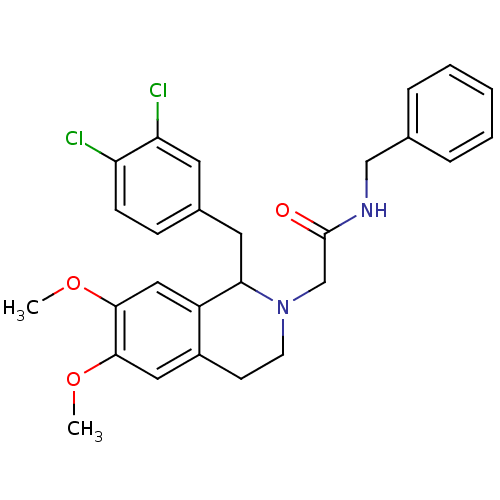
(CHEMBL489485 | N-benzyl-2-(1-(3,4-dichlorobenzyl)-...)Show SMILES COc1cc2CCN(CC(=O)NCc3ccccc3)C(Cc3ccc(Cl)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C27H28Cl2N2O3/c1-33-25-14-20-10-11-31(17-27(32)30-16-18-6-4-3-5-7-18)24(21(20)15-26(25)34-2)13-19-8-9-22(28)23(29)12-19/h3-9,12,14-15,24H,10-11,13,16-17H2,1-2H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50292928

((+/-)-N-benzyl-2-(1-(3,4-dimethoxybenzyl)-6,7-dime...)Show SMILES COc1ccc(CC2N(CC(=O)NCc3ccccc3)CCc3cc(OC)c(OC)cc23)cc1OC Show InChI InChI=1S/C29H34N2O5/c1-33-25-11-10-21(15-26(25)34-2)14-24-23-17-28(36-4)27(35-3)16-22(23)12-13-31(24)19-29(32)30-18-20-8-6-5-7-9-20/h5-11,15-17,24H,12-14,18-19H2,1-4H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50292939

(CHEMBL489485 | N-benzyl-2-(1-(3,4-dichlorobenzyl)-...)Show SMILES COc1cc2CCN(CC(=O)NCc3ccccc3)C(Cc3ccc(Cl)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C27H28Cl2N2O3/c1-33-25-14-20-10-11-31(17-27(32)30-16-18-6-4-3-5-7-18)24(21(20)15-26(25)34-2)13-19-8-9-22(28)23(29)12-19/h3-9,12,14-15,24H,10-11,13,16-17H2,1-2H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50092810

(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50028059

(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHO cells by FLIPR assay |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM154927

(US9000029, 6)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCC[C@H]1C(=O)Nc1cccc(SC)c1 Show InChI InChI=1S/C19H22N2O4S2/c1-25-15-8-10-17(11-9-15)27(23,24)21-12-4-7-18(21)19(22)20-14-5-3-6-16(13-14)26-2/h3,5-6,8-11,13,18H,4,7,12H2,1-2H3,(H,20,22)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50292928

((+/-)-N-benzyl-2-(1-(3,4-dimethoxybenzyl)-6,7-dime...)Show SMILES COc1ccc(CC2N(CC(=O)NCc3ccccc3)CCc3cc(OC)c(OC)cc23)cc1OC Show InChI InChI=1S/C29H34N2O5/c1-33-25-11-10-21(15-26(25)34-2)14-24-23-17-28(36-4)27(35-3)16-22(23)12-13-31(24)19-29(32)30-18-20-8-6-5-7-9-20/h5-11,15-17,24H,12-14,18-19H2,1-4H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50148576

(CHEMBL3771066)Show SMILES COc1ccc(CC2N(CC(=O)NCc3ccccc3)CCc3cc(OC)c(OC(C)C)cc23)cc1OC Show InChI InChI=1S/C31H38N2O5/c1-21(2)38-30-18-25-24(17-29(30)37-5)13-14-33(20-31(34)32-19-22-9-7-6-8-10-22)26(25)15-23-11-12-27(35-3)28(16-23)36-4/h6-12,16-18,21,26H,13-15,19-20H2,1-5H3,(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM154947

(US9000029, 26)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCC[C@H]1C(=O)Nc1cc(C)cc(C)c1 Show InChI InChI=1S/C20H24N2O4S/c1-14-11-15(2)13-16(12-14)21-20(23)19-5-4-10-22(19)27(24,25)18-8-6-17(26-3)7-9-18/h6-9,11-13,19H,4-5,10H2,1-3H3,(H,21,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data