 Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50047104
Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50047104 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC3 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC6 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC1 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC6 using acetylated p53 (379 to 382 residues) as substrate by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC1 using [3H]acetyl histone H4 peptide substrate incubated for 60 mins by scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC3 using [3H]acetyl histone H4 peptide substrate incubated for 60 mins by scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC6 using [3H]acetyl histone H4 peptide substrate incubated for 60 mins by scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50148630
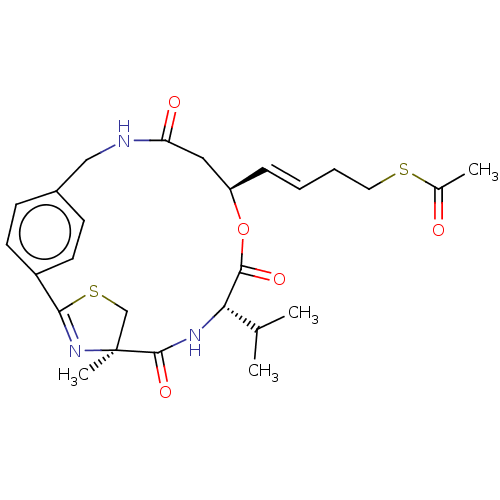
(CHEMBL3771206)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2ccc(CNC(=O)C[C@H](OC1=O)\C=C\CCSC(C)=O)cc2 |r,wU:22.27,wD:3.2,7.7,c:11,(-2.44,-5.97,;-3.02,-4.88,;-4.25,-4.85,;-2.21,-3.56,;-3.34,-2.53,;-4.03,-1.15,;-5.22,-1.48,;-4.17,.39,;-3.28,-.46,;-2.34,.31,;-1.66,1.69,;-2.82,3.1,;-3.75,1.87,;-1.51,3.91,;-.42,1.95,;1.1,1.67,;2.82,3.1,;3.75,1.87,;4.17,.39,;4.03,-1.15,;5.22,-1.48,;3.34,-2.53,;2.21,-3.56,;.77,-4.12,;-.77,-4.12,;-1,-5.33,;3.02,-4.88,;4.56,-4.83,;5.38,-6.14,;6.92,-6.09,;7.73,-7.4,;9.27,-7.35,;9.85,-6.26,;9.92,-8.39,;1.51,3.91,;,4.19,)| Show InChI InChI=1S/C26H33N3O5S2/c1-16(2)22-24(32)34-20(7-5-6-12-35-17(3)30)13-21(31)27-14-18-8-10-19(11-9-18)23-29-26(4,15-36-23)25(33)28-22/h5,7-11,16,20,22H,6,12-15H2,1-4H3,(H,27,31)(H,28,33)/b7-5+/t20-,22+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC3 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50397360

(CHEMBL2170177 | US10188756, Compound CN110)Show InChI InChI=1S/C17H16N2O3/c1-22-15-6-2-12(3-7-15)11-19-9-8-13-4-5-14(10-16(13)19)17(20)18-21/h2-10,21H,11H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC8 preincubated for 15 mins followed by acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin substrate addition measured after... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC8 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC3/NcoR2 using acetylated p53 (379 to 382 residues) as substrate by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDAC3 in human K562 cells using [3H]acetylated histone as substrate incubated for 10 mins by liquid scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50121210
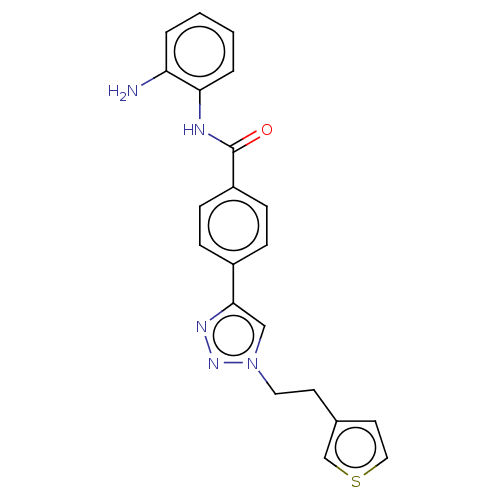
(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC1 by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50322422
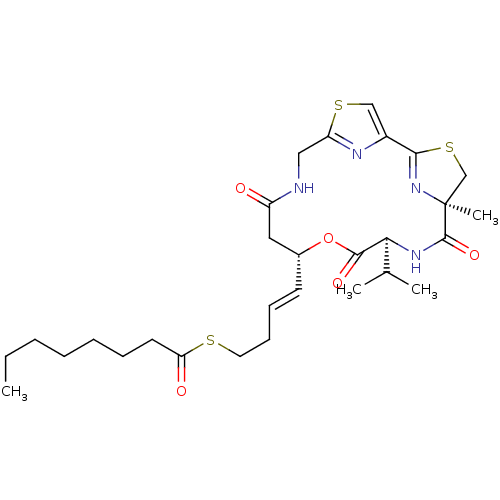
(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant KDAC3 using fluorophore-conjugated substrate measured as fluorigenic release of 7-amino-4-methylcoumarin ... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDAC1 in human K562 cells using [3H]acetylated histone as substrate incubated for 10 mins by liquid scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50148630

(CHEMBL3771206)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2ccc(CNC(=O)C[C@H](OC1=O)\C=C\CCSC(C)=O)cc2 |r,wU:22.27,wD:3.2,7.7,c:11,(-2.44,-5.97,;-3.02,-4.88,;-4.25,-4.85,;-2.21,-3.56,;-3.34,-2.53,;-4.03,-1.15,;-5.22,-1.48,;-4.17,.39,;-3.28,-.46,;-2.34,.31,;-1.66,1.69,;-2.82,3.1,;-3.75,1.87,;-1.51,3.91,;-.42,1.95,;1.1,1.67,;2.82,3.1,;3.75,1.87,;4.17,.39,;4.03,-1.15,;5.22,-1.48,;3.34,-2.53,;2.21,-3.56,;.77,-4.12,;-.77,-4.12,;-1,-5.33,;3.02,-4.88,;4.56,-4.83,;5.38,-6.14,;6.92,-6.09,;7.73,-7.4,;9.27,-7.35,;9.85,-6.26,;9.92,-8.39,;1.51,3.91,;,4.19,)| Show InChI InChI=1S/C26H33N3O5S2/c1-16(2)22-24(32)34-20(7-5-6-12-35-17(3)30)13-21(31)27-14-18-8-10-19(11-9-18)23-29-26(4,15-36-23)25(33)28-22/h5,7-11,16,20,22H,6,12-15H2,1-4H3,(H,27,31)(H,28,33)/b7-5+/t20-,22+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC6 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50322422

(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant KDAC1 using fluorophore-conjugated substrate measured as fluorigenic release of 7-amino-4-methylcoumarin ... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC8 using diacetylated p53 (379 to 382 residues) as substrate by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC1 using acetylated p53 (379 to 382 residues) as substrate by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50148630

(CHEMBL3771206)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2ccc(CNC(=O)C[C@H](OC1=O)\C=C\CCSC(C)=O)cc2 |r,wU:22.27,wD:3.2,7.7,c:11,(-2.44,-5.97,;-3.02,-4.88,;-4.25,-4.85,;-2.21,-3.56,;-3.34,-2.53,;-4.03,-1.15,;-5.22,-1.48,;-4.17,.39,;-3.28,-.46,;-2.34,.31,;-1.66,1.69,;-2.82,3.1,;-3.75,1.87,;-1.51,3.91,;-.42,1.95,;1.1,1.67,;2.82,3.1,;3.75,1.87,;4.17,.39,;4.03,-1.15,;5.22,-1.48,;3.34,-2.53,;2.21,-3.56,;.77,-4.12,;-.77,-4.12,;-1,-5.33,;3.02,-4.88,;4.56,-4.83,;5.38,-6.14,;6.92,-6.09,;7.73,-7.4,;9.27,-7.35,;9.85,-6.26,;9.92,-8.39,;1.51,3.91,;,4.19,)| Show InChI InChI=1S/C26H33N3O5S2/c1-16(2)22-24(32)34-20(7-5-6-12-35-17(3)30)13-21(31)27-14-18-8-10-19(11-9-18)23-29-26(4,15-36-23)25(33)28-22/h5,7-11,16,20,22H,6,12-15H2,1-4H3,(H,27,31)(H,28,33)/b7-5+/t20-,22+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC1 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC3 by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC8 using [3H]acetyl histone H4 peptide substrate incubated for 60 mins by scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50397360

(CHEMBL2170177 | US10188756, Compound CN110)Show InChI InChI=1S/C17H16N2O3/c1-22-15-6-2-12(3-7-15)11-19-9-8-13-4-5-14(10-16(13)19)17(20)18-21/h2-10,21H,11H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC6 preincubated for 15 mins followed by acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin substrate addition measured after... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50148629
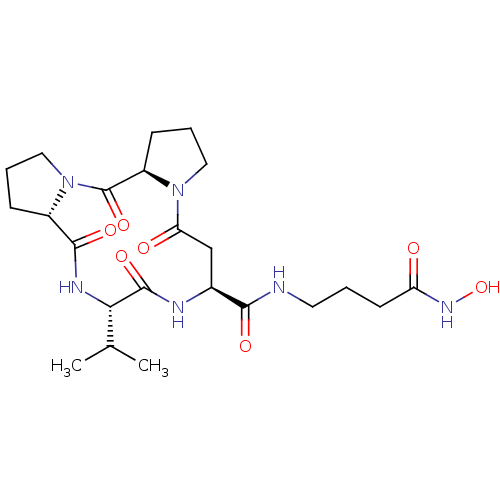
(CHEMBL3769491)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)C[C@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC6 using FITC-histone 4 acetylated peptide substrate incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50148628
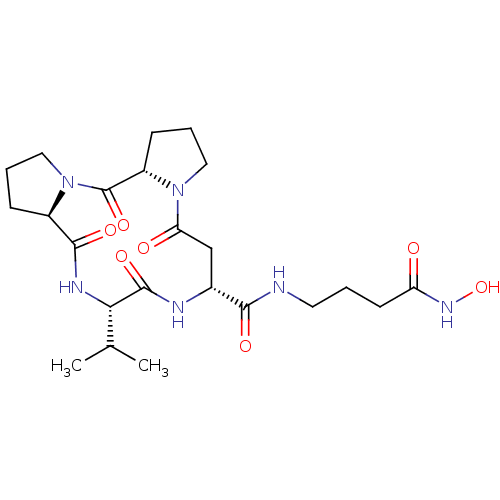
(CHEMBL3769629)Show SMILES [H][C@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)C[C@@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC6 using FITC-histone 4 acetylated peptide substrate incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50322422

(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant KDAC6 using fluorophore-conjugated substrate measured as fluorigenic release of 7-amino-4-methylcoumarin ... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50148629

(CHEMBL3769491)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)C[C@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC3 using FITC-p53 acetylated peptide substrate incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50397360

(CHEMBL2170177 | US10188756, Compound CN110)Show InChI InChI=1S/C17H16N2O3/c1-22-15-6-2-12(3-7-15)11-19-9-8-13-4-5-14(10-16(13)19)17(20)18-21/h2-10,21H,11H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC1 preincubated for 15 mins followed by acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin substrate addition measured after... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50148629

(CHEMBL3769491)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)C[C@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC1 using FAM-labelled substrate A incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDAC8 in human K562 cells using [3H]acetylated histone as substrate incubated for 10 mins by liquid scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDAC6 in human K562 cells using [3H]acetylated histone as substrate incubated for 10 mins by liquid scintillation counting method |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50148628

(CHEMBL3769629)Show SMILES [H][C@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)C[C@@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC1 using FAM-labelled substrate A incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50148628

(CHEMBL3769629)Show SMILES [H][C@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)C[C@@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC8 using FAM-labelled substrate B incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50148629

(CHEMBL3769491)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)C[C@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC8 using FAM-labelled substrate B incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50148628

(CHEMBL3769629)Show SMILES [H][C@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)C[C@@H](NC(=O)[C@@H](NC2=O)C(C)C)C(=O)NCCCC(=O)NO |r| Show InChI InChI=1S/C23H36N6O7/c1-13(2)19-22(34)25-14(20(32)24-9-3-8-17(30)27-36)12-18(31)28-10-5-7-16(28)23(35)29-11-4-6-15(29)21(33)26-19/h13-16,19,36H,3-12H2,1-2H3,(H,24,32)(H,25,34)(H,26,33)(H,27,30)/t14-,15-,16+,19+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human KDAC3 using FITC-p53 acetylated peptide substrate incubated for 60 mins by microfluidic chip-based assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC8 by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50322422

(CHEMBL1173445 | Largazole)Show SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26| Show InChI InChI=1S/C29H42N4O5S3/c1-5-6-7-8-9-13-24(35)39-14-11-10-12-20-15-22(34)30-16-23-31-21(17-40-23)26-33-29(4,18-41-26)28(37)32-25(19(2)3)27(36)38-20/h10,12,17,19-20,25H,5-9,11,13-16,18H2,1-4H3,(H,30,34)(H,32,37)/b12-10+/t20-,25+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant KDAC8 using fluorophore-conjugated substrate measured as fluorigenic release of 7-amino-4-methylcoumarin ... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50148630

(CHEMBL3771206)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2ccc(CNC(=O)C[C@H](OC1=O)\C=C\CCSC(C)=O)cc2 |r,wU:22.27,wD:3.2,7.7,c:11,(-2.44,-5.97,;-3.02,-4.88,;-4.25,-4.85,;-2.21,-3.56,;-3.34,-2.53,;-4.03,-1.15,;-5.22,-1.48,;-4.17,.39,;-3.28,-.46,;-2.34,.31,;-1.66,1.69,;-2.82,3.1,;-3.75,1.87,;-1.51,3.91,;-.42,1.95,;1.1,1.67,;2.82,3.1,;3.75,1.87,;4.17,.39,;4.03,-1.15,;5.22,-1.48,;3.34,-2.53,;2.21,-3.56,;.77,-4.12,;-.77,-4.12,;-1,-5.33,;3.02,-4.88,;4.56,-4.83,;5.38,-6.14,;6.92,-6.09,;7.73,-7.4,;9.27,-7.35,;9.85,-6.26,;9.92,-8.39,;1.51,3.91,;,4.19,)| Show InChI InChI=1S/C26H33N3O5S2/c1-16(2)22-24(32)34-20(7-5-6-12-35-17(3)30)13-21(31)27-14-18-8-10-19(11-9-18)23-29-26(4,15-36-23)25(33)28-22/h5,7-11,16,20,22H,6,12-15H2,1-4H3,(H,27,31)(H,28,33)/b7-5+/t20-,22+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC8 |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC6 by fluorescence assay |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50397360

(CHEMBL2170177 | US10188756, Compound CN110)Show InChI InChI=1S/C17H16N2O3/c1-22-15-6-2-12(3-7-15)11-19-9-8-13-4-5-14(10-16(13)19)17(20)18-21/h2-10,21H,11H2,1H3,(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human KDAC3 preincubated for 15 mins followed by acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin substrate addition measured after... |
J Med Chem 59: 1613-33 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01632
BindingDB Entry DOI: 10.7270/Q2SB47MP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data