

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284986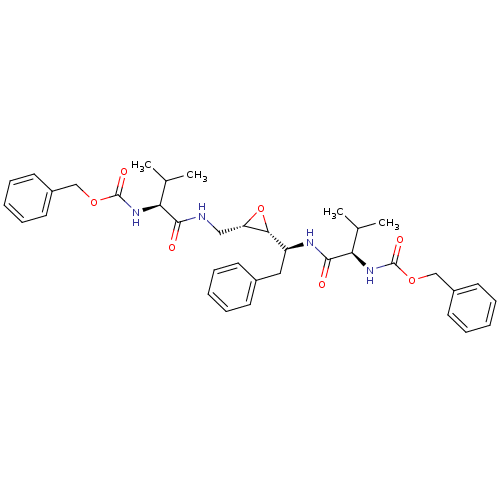 (CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50009242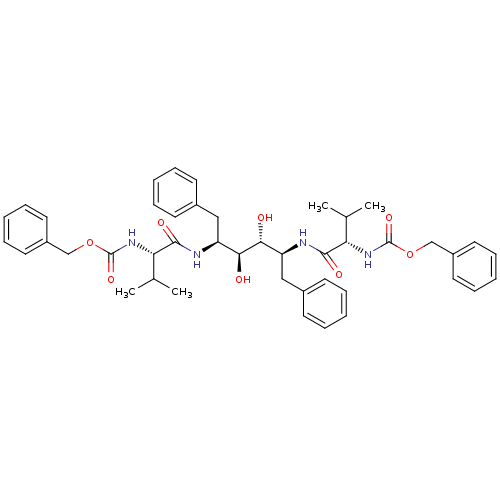 (CHEMBL48565 | {(S)-1-[(1S,2S,3R,4S)-1-Benzyl-4-((S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197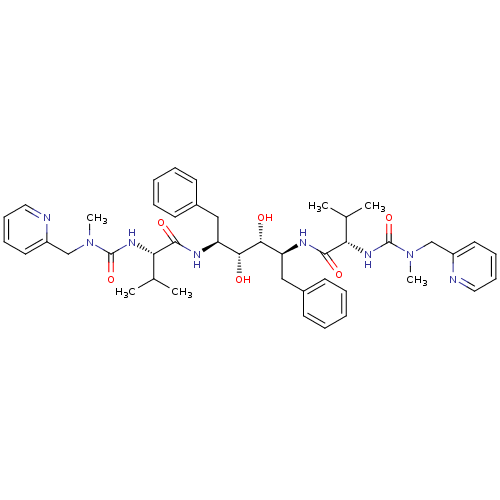 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284988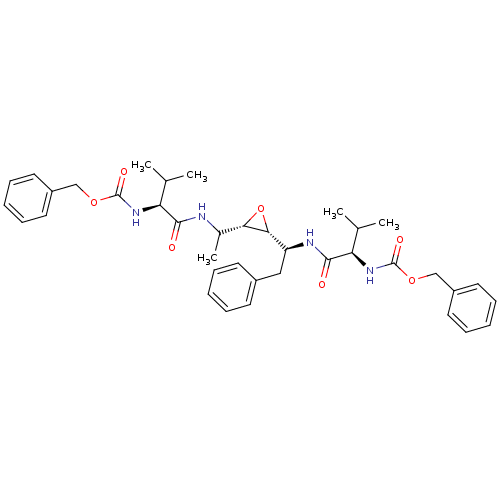 (CHEMBL52809 | [(S)-1-(1-{(2S,3R)-3-[(S)-1-((R)-2-B...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284989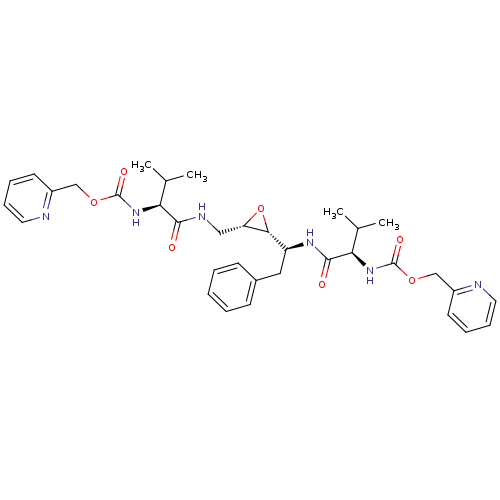 (CHEMBL300705 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284984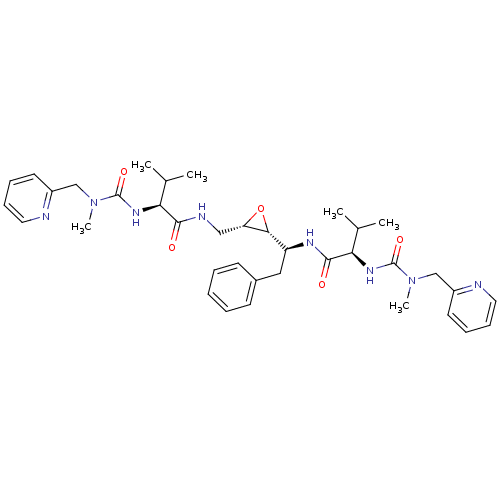 ((R)-3-Methyl-N-[(S)-1-((2R,3S)-3-{[(S)-3-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284987 (CHEMBL293061 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284991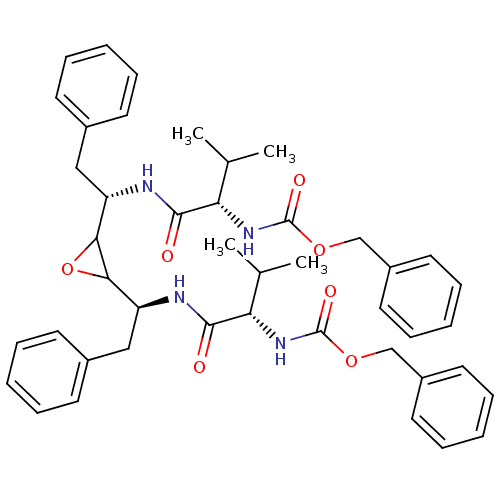 (CHEMBL295499 | [(S)-1-((S)-1-{3-[(S)-1-((S)-2-Benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284990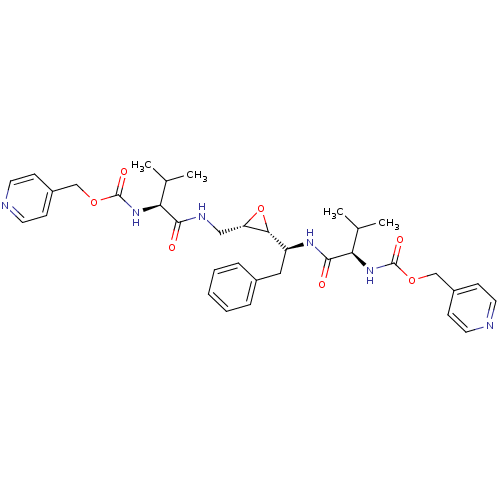 (CHEMBL54394 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284985 (1,2-Epoxy-3-(4'-nitrophenoxy)propane | 1,2-Epoxy-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents | Article | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||