

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50289727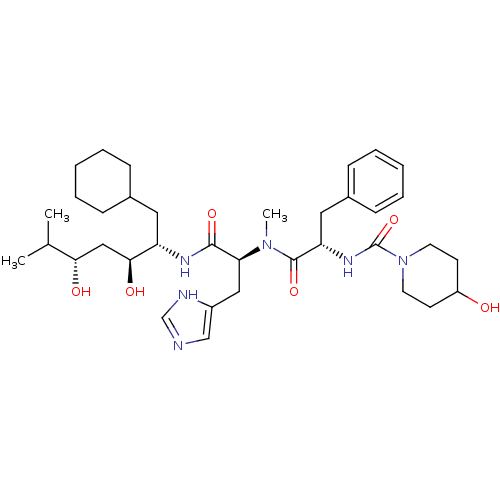 (4-Hydroxy-piperidine-1-carboxylic acid ((S)-1-{[(S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289726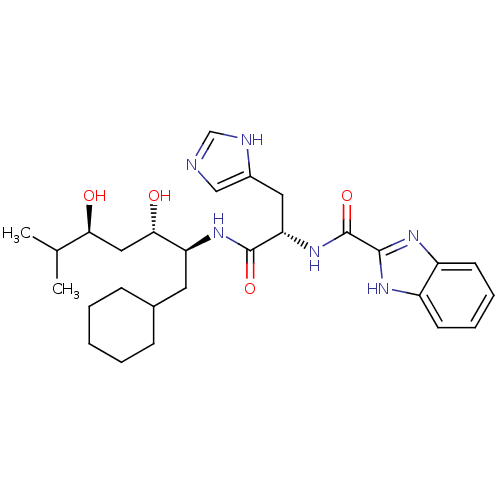 (1H-Benzoimidazole-2-carboxylic acid [(S)-1-((1S,2S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289714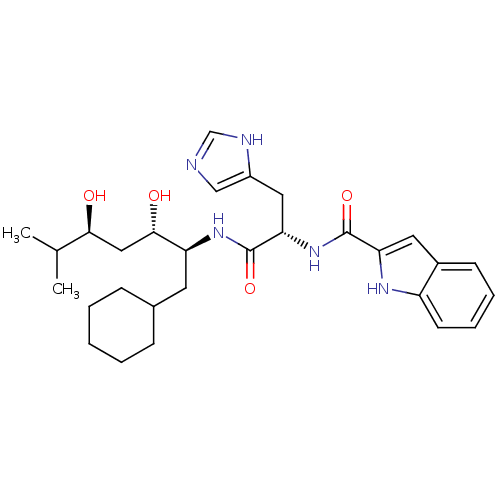 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of marmoset plasma renin, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289716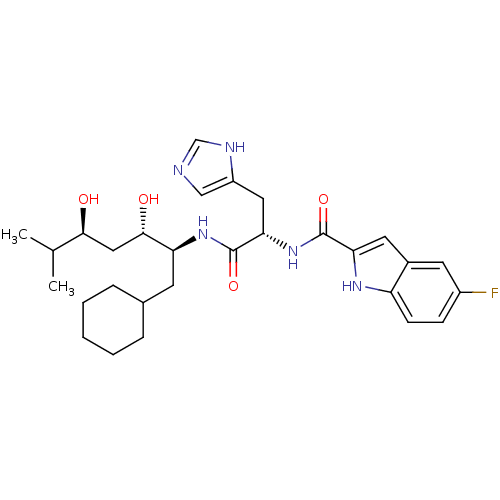 (5-Fluoro-1H-indole-2-carboxylic acid [(S)-1-((1S,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289724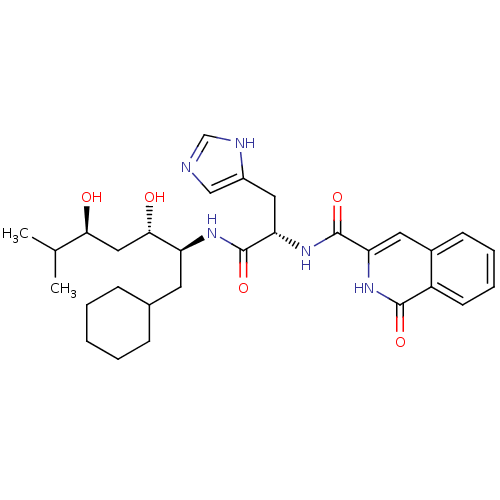 (1-Oxo-1,2-dihydro-isoquinoline-3-carboxylic acid [...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of dog plasma renin, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289723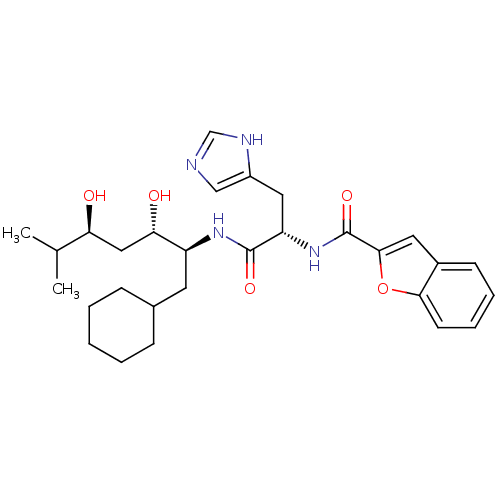 (Benzofuran-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289720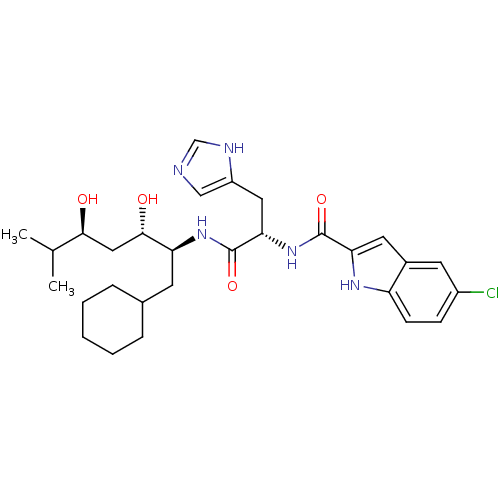 (5-Chloro-1H-indole-2-carboxylic acid [(S)-1-((1S,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289722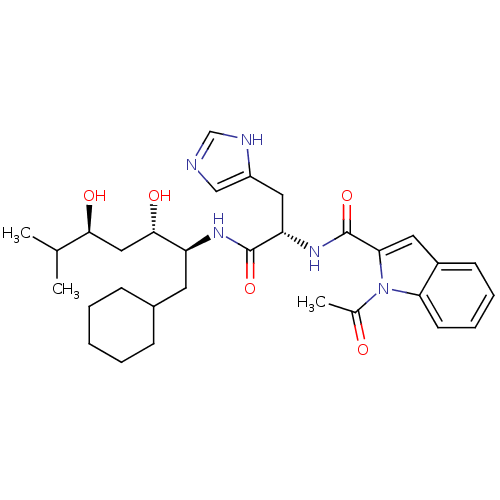 (1-Acetyl-1H-indole-2-carboxylic acid [(S)-1-((1S,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289721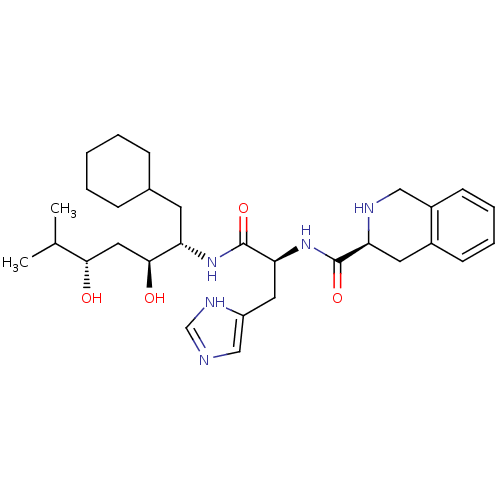 ((S)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289718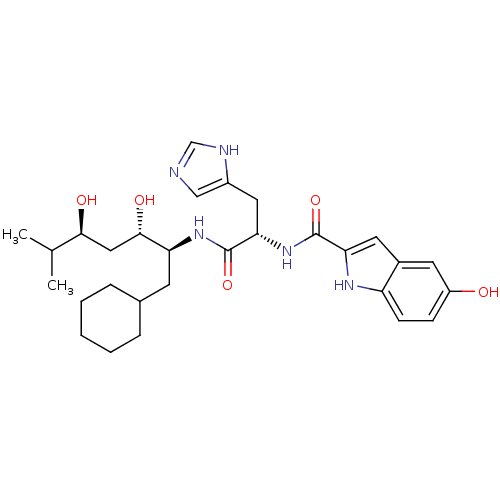 (5-Hydroxy-1H-indole-2-carboxylic acid [(S)-1-((1S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289725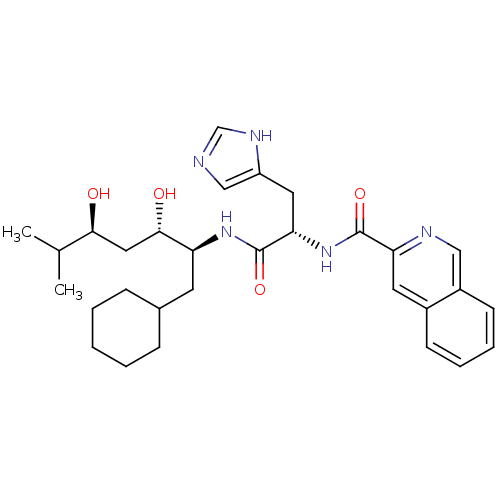 (CHEMBL301306 | Isoquinoline-3-carboxylic acid [(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289715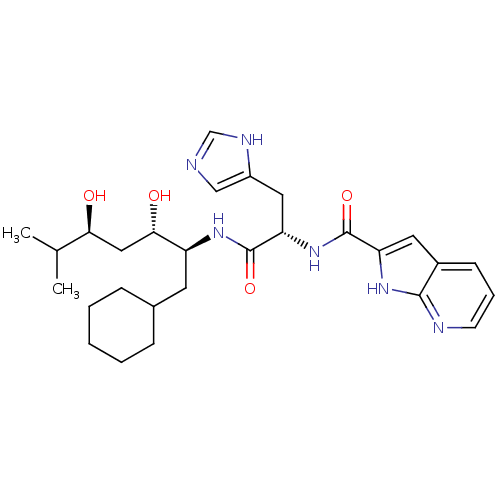 (1H-Pyrrolo[2,3-b]pyridine-2-carboxylic acid [(S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289713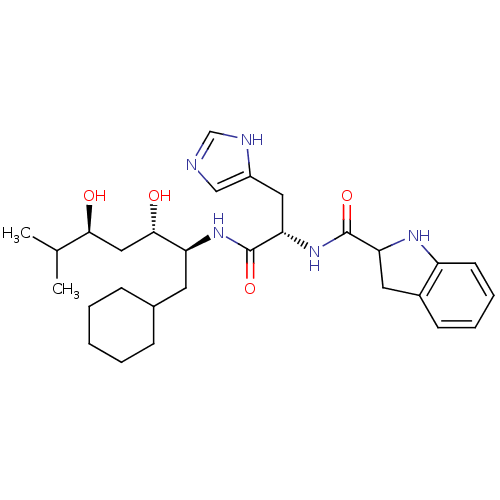 (2,3-Dihydro-1H-indole-2-carboxylic acid [(S)-1-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289713 (2,3-Dihydro-1H-indole-2-carboxylic acid [(S)-1-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of monkey plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289719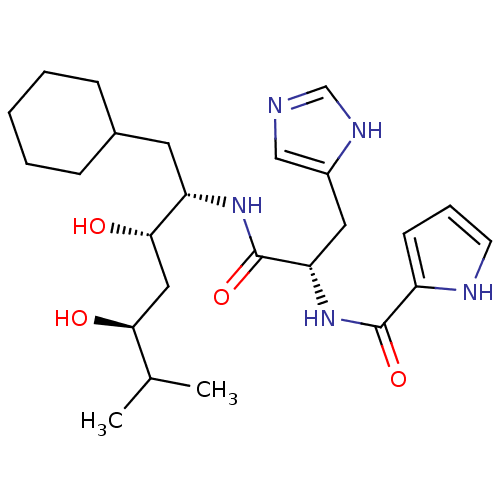 (1H-Pyrrole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50289717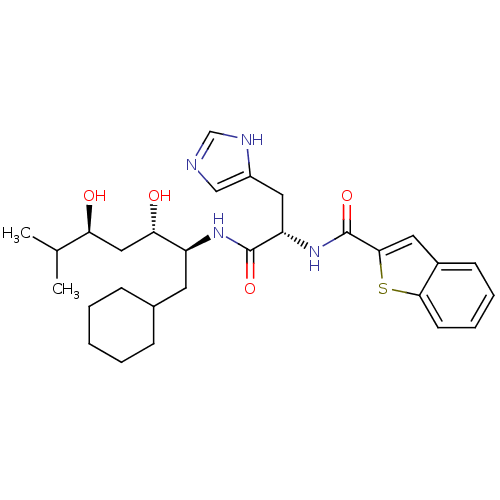 (Benzo[b]thiophene-2-carboxylic acid [(S)-1-((1S,2S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin D (bovine), expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against ACE human, expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory effect of the compound was evaluated against pepsin, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50289714 (1H-Indole-2-carboxylic acid [(S)-1-((1S,2S,4S)-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of rat plasma renin, Expressed as IC50 | Bioorg Med Chem Lett 7: 1863-1868 (1997) Article DOI: 10.1016/S0960-894X(97)00323-5 BindingDB Entry DOI: 10.7270/Q2K64J20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||