 Found 149 hits Enz. Inhib. hit(s) with all data for entry = 50047712
Found 149 hits Enz. Inhib. hit(s) with all data for entry = 50047712 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50062357

(AP26113 | CHEMBL3397300)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N(C)C Show InChI InChI=1S/C26H34ClN6O2P/c1-32(2)18-12-14-33(15-13-18)19-10-11-21(23(16-19)35-3)30-26-28-17-20(27)25(31-26)29-22-8-6-7-9-24(22)36(4,5)34/h6-11,16-18H,12-15H2,1-5H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185287
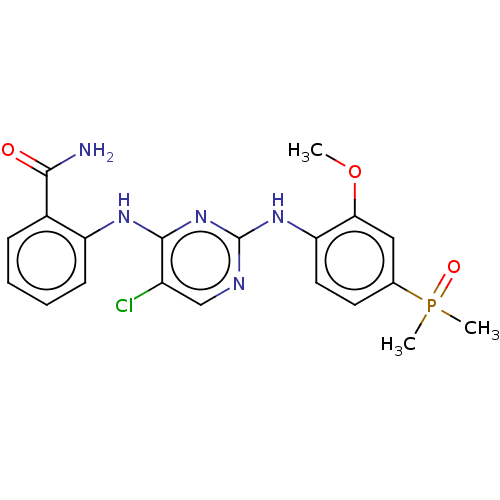
(CHEMBL3823268)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H21ClN5O3P/c1-29-17-10-12(30(2,3)28)8-9-16(17)25-20-23-11-14(21)19(26-20)24-15-7-5-4-6-13(15)18(22)27/h4-11H,1-3H3,(H2,22,27)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185237

(CHEMBL3824308)Show SMILES CCP(=O)(CC)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C31H43ClN7O2P/c1-5-42(40,6-2)29-10-8-7-9-27(29)34-30-25(32)22-33-31(36-30)35-26-12-11-24(21-28(26)41-4)38-15-13-23(14-16-38)39-19-17-37(3)18-20-39/h7-12,21-23H,5-6,13-20H2,1-4H3,(H2,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM482158
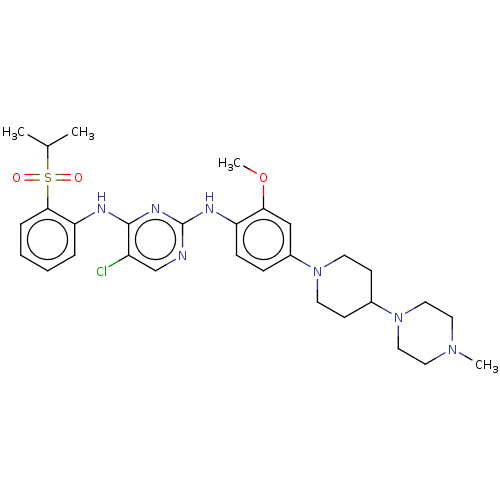
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185280

(CHEMBL3822611)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H30ClN6O2P/c1-30-11-13-31(14-12-30)17-9-10-19(21(15-17)33-2)28-24-26-16-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)32/h5-10,15-16H,11-14H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185275
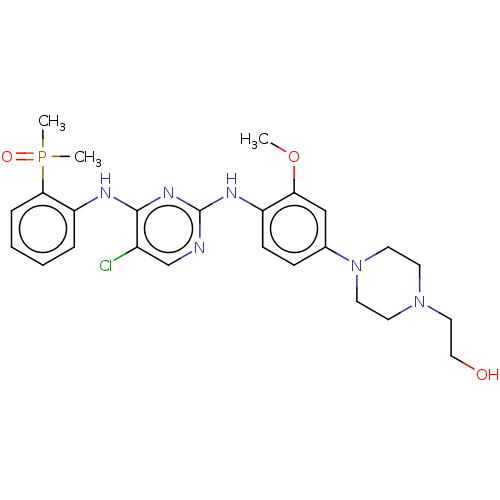
(CHEMBL3823235)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C25H32ClN6O3P/c1-35-22-16-18(32-12-10-31(11-13-32)14-15-33)8-9-20(22)29-25-27-17-19(26)24(30-25)28-21-6-4-5-7-23(21)36(2,3)34/h4-9,16-17,33H,10-15H2,1-3H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185284
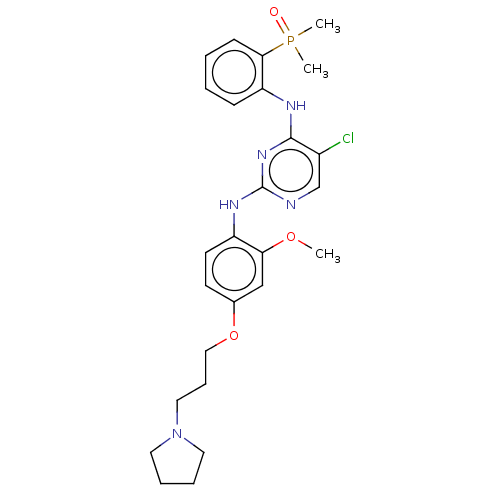
(CHEMBL3823549)Show SMILES COc1cc(OCCCN2CCCC2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C26H33ClN5O3P/c1-34-23-17-19(35-16-8-15-32-13-6-7-14-32)11-12-21(23)30-26-28-18-20(27)25(31-26)29-22-9-4-5-10-24(22)36(2,3)33/h4-5,9-12,17-18H,6-8,13-16H2,1-3H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185281
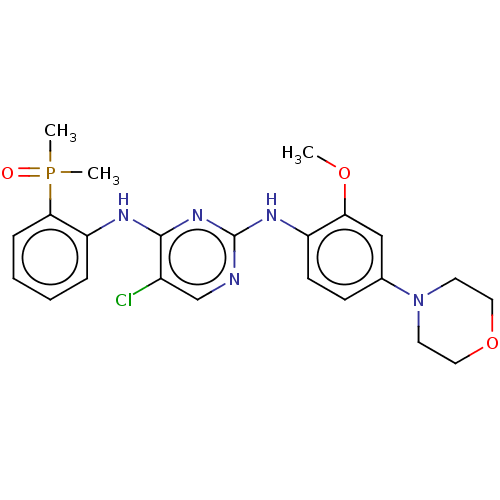
(CHEMBL3823416)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCOCC1 Show InChI InChI=1S/C23H27ClN5O3P/c1-31-20-14-16(29-10-12-32-13-11-29)8-9-18(20)27-23-25-15-17(24)22(28-23)26-19-6-4-5-7-21(19)33(2,3)30/h4-9,14-15H,10-13H2,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185268

(CHEMBL3824327)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CC1)C1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-13-11-21(12-14-35)36-15-17-37(18-16-36)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185290
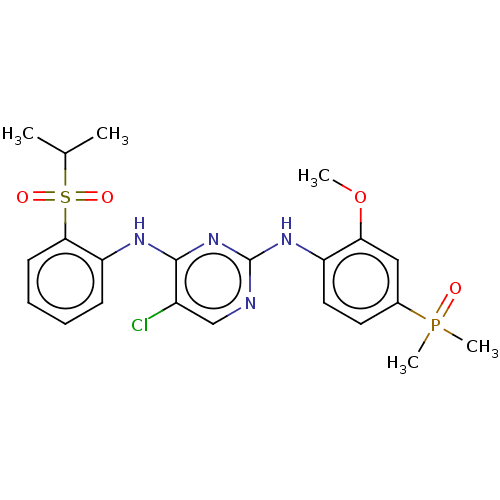
(CHEMBL3824304)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)P(C)(C)=O Show InChI InChI=1S/C22H26ClN4O4PS/c1-14(2)33(29,30)20-9-7-6-8-18(20)25-21-16(23)13-24-22(27-21)26-17-11-10-15(32(4,5)28)12-19(17)31-3/h6-14H,1-5H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185286
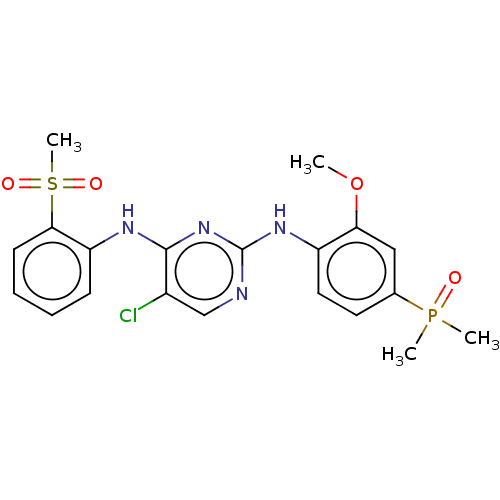
(CHEMBL3823256)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H22ClN4O4PS/c1-29-17-11-13(30(2,3)26)9-10-15(17)24-20-22-12-14(21)19(25-20)23-16-7-5-6-8-18(16)31(4,27)28/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185272
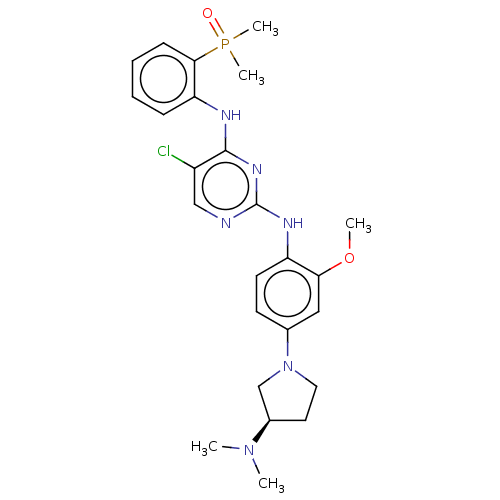
(CHEMBL3823107)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CC[C@H](C1)N(C)C |r| Show InChI InChI=1S/C25H32ClN6O2P/c1-31(2)18-12-13-32(16-18)17-10-11-20(22(14-17)34-3)29-25-27-15-19(26)24(30-25)28-21-8-6-7-9-23(21)35(4,5)33/h6-11,14-15,18H,12-13,16H2,1-5H3,(H2,27,28,29,30)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185283

(CHEMBL3823603)Show SMILES COc1cc(OC2CCN(C)C2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C24H29ClN5O3P/c1-30-12-11-17(15-30)33-16-9-10-19(21(13-16)32-2)28-24-26-14-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)31/h5-10,13-14,17H,11-12,15H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM482158

(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185278

(CHEMBL3823017)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(O)CC1 Show InChI InChI=1S/C24H29ClN5O3P/c1-33-21-14-16(30-12-10-17(31)11-13-30)8-9-19(21)28-24-26-15-18(25)23(29-24)27-20-6-4-5-7-22(20)34(2,3)32/h4-9,14-15,17,31H,10-13H2,1-3H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185140
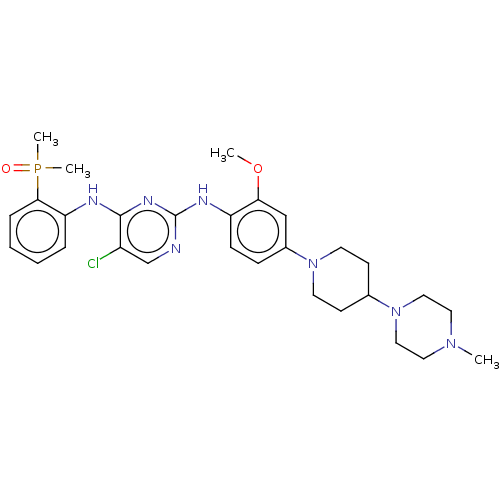
(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185245

(CHEMBL3823190)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)C1CCN(C)CC1 Show InChI InChI=1S/C25H31ClN5O2P/c1-31-13-11-17(12-14-31)18-9-10-20(22(15-18)33-2)29-25-27-16-19(26)24(30-25)28-21-7-5-6-8-23(21)34(3,4)32/h5-10,15-17H,11-14H2,1-4H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185239
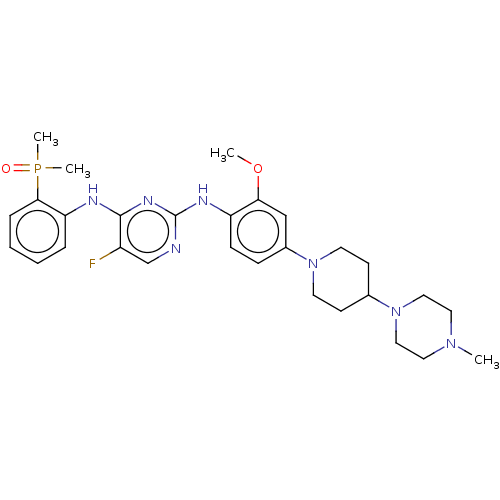
(CHEMBL3823296)Show SMILES COc1cc(ccc1Nc1ncc(F)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39FN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185286

(CHEMBL3823256)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H22ClN4O4PS/c1-29-17-11-13(30(2,3)26)9-10-15(17)24-20-22-12-14(21)19(25-20)23-16-7-5-6-8-18(16)31(4,27)28/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185238

(CHEMBL3823577)Show SMILES COc1cc(ccc1Nc1ncc(C)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H42N7O2P/c1-22-21-31-30(34-29(22)32-26-8-6-7-9-28(26)40(4,5)38)33-25-11-10-24(20-27(25)39-3)36-14-12-23(13-15-36)37-18-16-35(2)17-19-37/h6-11,20-21,23H,12-19H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074
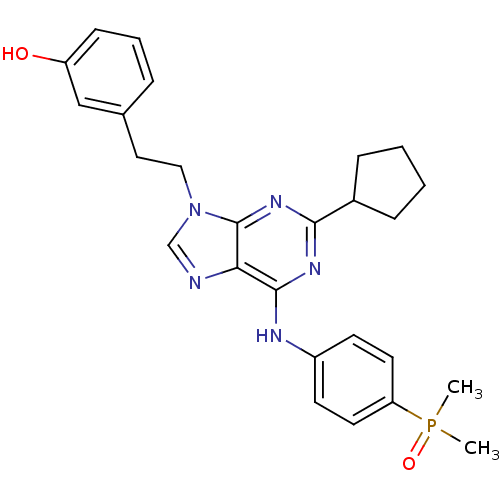
(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 6x-His-tagged human Src kinase domain (T250 to L536 residues) expressed in Sf9 cells incubated for 2 hrs in presence of biotinylated cd... |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185288

(CHEMBL3824326)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)=O)n1)P(C)(C)=O Show InChI InChI=1S/C21H22ClN4O3P/c1-13(27)15-7-5-6-8-17(15)24-20-16(22)12-23-21(26-20)25-18-10-9-14(30(3,4)28)11-19(18)29-2/h5-12H,1-4H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185265
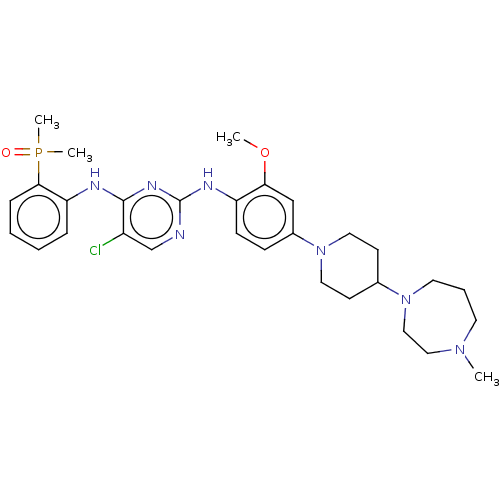
(CHEMBL3824290)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCCN(C)CC1 Show InChI InChI=1S/C30H41ClN7O2P/c1-36-14-7-15-37(19-18-36)22-12-16-38(17-13-22)23-10-11-25(27(20-23)40-2)34-30-32-21-24(31)29(35-30)33-26-8-5-6-9-28(26)41(3,4)39/h5-6,8-11,20-22H,7,12-19H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185267
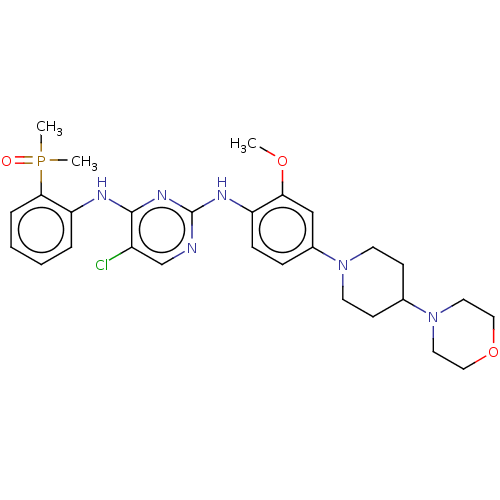
(CHEMBL3822557)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C28H36ClN6O3P/c1-37-25-18-21(34-12-10-20(11-13-34)35-14-16-38-17-15-35)8-9-23(25)32-28-30-19-22(29)27(33-28)31-24-6-4-5-7-26(24)39(2,3)36/h4-9,18-20H,10-17H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185270
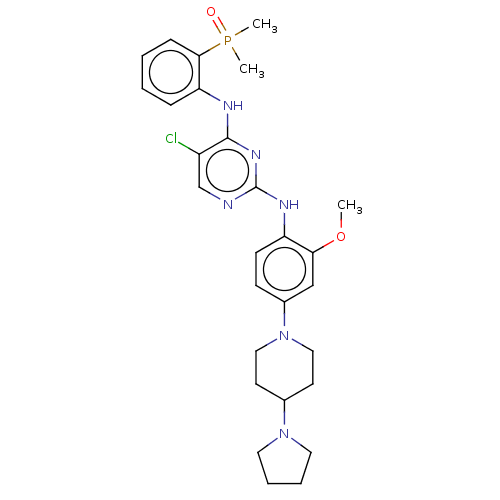
(CHEMBL3823031)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCCC1 Show InChI InChI=1S/C28H36ClN6O2P/c1-37-25-18-21(35-16-12-20(13-17-35)34-14-6-7-15-34)10-11-23(25)32-28-30-19-22(29)27(33-28)31-24-8-4-5-9-26(24)38(2,3)36/h4-5,8-11,18-20H,6-7,12-17H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185282
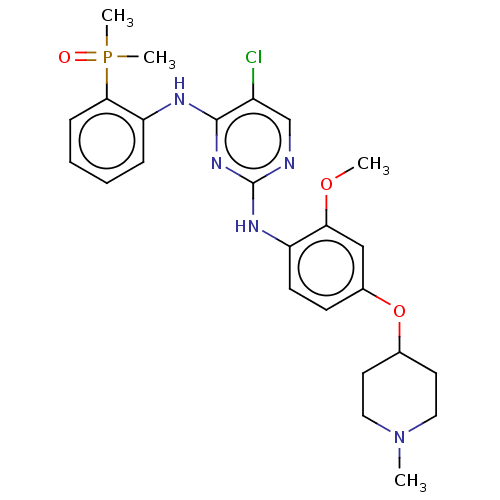
(CHEMBL3823916)Show SMILES COc1cc(OC2CCN(C)CC2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C25H31ClN5O3P/c1-31-13-11-17(12-14-31)34-18-9-10-20(22(15-18)33-2)29-25-27-16-19(26)24(30-25)28-21-7-5-6-8-23(21)35(3,4)32/h5-10,15-17H,11-14H2,1-4H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185279

(CHEMBL3824007)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C25H30ClN6O3P/c1-17(33)31-11-13-32(14-12-31)18-9-10-20(22(15-18)35-2)29-25-27-16-19(26)24(30-25)28-21-7-5-6-8-23(21)36(3,4)34/h5-10,15-16H,11-14H2,1-4H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185152
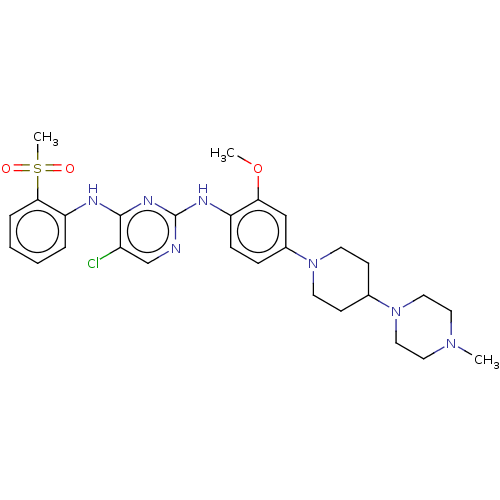
(CHEMBL3822475)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C28H36ClN7O3S/c1-34-14-16-36(17-15-34)20-10-12-35(13-11-20)21-8-9-23(25(18-21)39-2)32-28-30-19-22(29)27(33-28)31-24-6-4-5-7-26(24)40(3,37)38/h4-9,18-20H,10-17H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185287

(CHEMBL3823268)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H21ClN5O3P/c1-29-17-10-12(30(2,3)28)8-9-16(17)25-20-23-11-14(21)19(26-20)24-15-7-5-4-6-13(15)18(22)27/h4-11H,1-3H3,(H2,22,27)(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185233
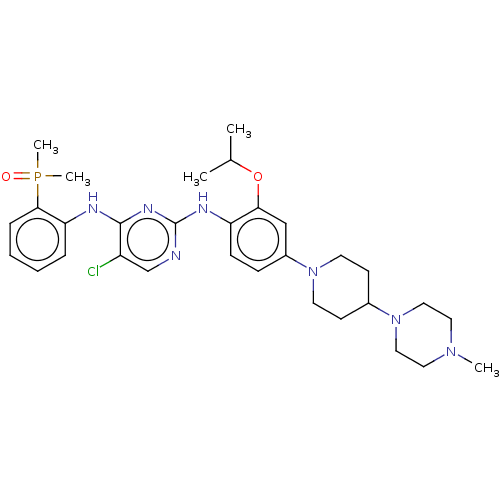
(CHEMBL3823224)Show SMILES CC(C)Oc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C31H43ClN7O2P/c1-22(2)41-28-20-24(38-14-12-23(13-15-38)39-18-16-37(3)17-19-39)10-11-26(28)35-31-33-21-25(32)30(36-31)34-27-8-6-7-9-29(27)42(4,5)40/h6-11,20-23H,12-19H2,1-5H3,(H2,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185274

(CHEMBL3824130)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCCN(C)CC1 Show InChI InChI=1S/C25H32ClN6O2P/c1-31-12-7-13-32(15-14-31)18-10-11-20(22(16-18)34-2)29-25-27-17-19(26)24(30-25)28-21-8-5-6-9-23(21)35(3,4)33/h5-6,8-11,16-17H,7,12-15H2,1-4H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185234
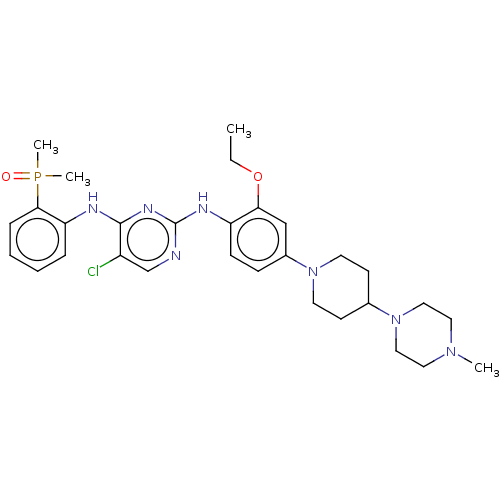
(CHEMBL3824318)Show SMILES CCOc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H41ClN7O2P/c1-5-40-27-20-23(37-14-12-22(13-15-37)38-18-16-36(2)17-19-38)10-11-25(27)34-30-32-21-24(31)29(35-30)33-26-8-6-7-9-28(26)41(3,4)39/h6-11,20-22H,5,12-19H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185161

(CHEMBL3822672)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)N(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN8O3S/c1-35(2)42(39,40)27-8-6-5-7-25(27)32-28-23(30)20-31-29(34-28)33-24-10-9-22(19-26(24)41-4)37-13-11-21(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185152

(CHEMBL3822475)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C28H36ClN7O3S/c1-34-14-16-36(17-15-34)20-10-12-35(13-11-20)21-8-9-23(25(18-21)39-2)32-28-30-19-22(29)27(33-28)31-24-6-4-5-7-26(24)40(3,37)38/h4-9,18-20H,10-17H2,1-3H3,(H2,30,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185246

(CHEMBL3822645)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C27H31ClN7O2P/c1-37-24-14-18(19-15-31-35(17-19)20-10-12-29-13-11-20)8-9-22(24)33-27-30-16-21(28)26(34-27)32-23-6-4-5-7-25(23)38(2,3)36/h4-9,14-17,20,29H,10-13H2,1-3H3,(H2,30,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185288

(CHEMBL3824326)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)=O)n1)P(C)(C)=O Show InChI InChI=1S/C21H22ClN4O3P/c1-13(27)15-7-5-6-8-17(15)24-20-16(22)12-23-21(26-20)25-18-10-9-14(30(3,4)28)11-19(18)29-2/h5-12H,1-4H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185163

(CHEMBL3824246)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C28H35ClN8O2/c1-35-13-15-37(16-14-35)19-9-11-36(12-10-19)20-7-8-24(25(17-20)39-2)33-28-31-18-22(29)27(34-28)32-23-6-4-3-5-21(23)26(30)38/h3-8,17-19H,9-16H2,1-2H3,(H2,30,38)(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185235
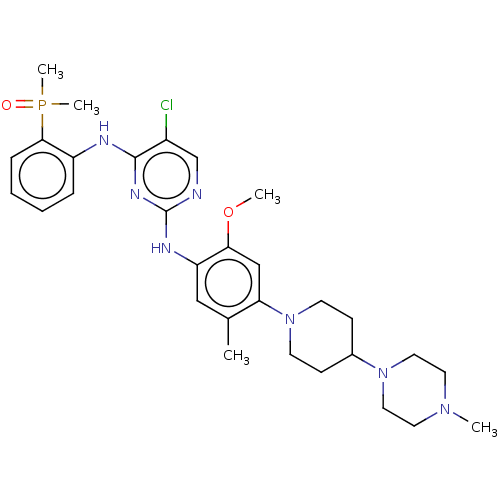
(CHEMBL3823836 | US20230322822, Control compound 2)Show SMILES COc1cc(N2CCC(CC2)N2CCN(C)CC2)c(C)cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C30H41ClN7O2P/c1-21-18-25(27(40-3)19-26(21)38-12-10-22(11-13-38)37-16-14-36(2)15-17-37)34-30-32-20-23(31)29(35-30)33-24-8-6-7-9-28(24)41(4,5)39/h6-9,18-20,22H,10-17H2,1-5H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185247

(CHEMBL3823243)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(C1)N1CCCCC1 Show InChI InChI=1S/C28H36ClN6O2P/c1-37-25-17-20(35-16-13-21(19-35)34-14-7-4-8-15-34)11-12-23(25)32-28-30-18-22(29)27(33-28)31-24-9-5-6-10-26(24)38(2,3)36/h5-6,9-12,17-18,21H,4,7-8,13-16,19H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185271

(CHEMBL3823218)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CN2CCC2)CC1 Show InChI InChI=1S/C28H36ClN6O2P/c1-37-25-17-21(35-15-11-20(12-16-35)19-34-13-6-14-34)9-10-23(25)32-28-30-18-22(29)27(33-28)31-24-7-4-5-8-26(24)38(2,3)36/h4-5,7-10,17-18,20H,6,11-16,19H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50185288

(CHEMBL3824326)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)=O)n1)P(C)(C)=O Show InChI InChI=1S/C21H22ClN4O3P/c1-13(27)15-7-5-6-8-17(15)24-20-16(22)12-23-21(26-20)25-18-10-9-14(30(3,4)28)11-19(18)29-2/h5-12H,1-4H3,(H2,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185289
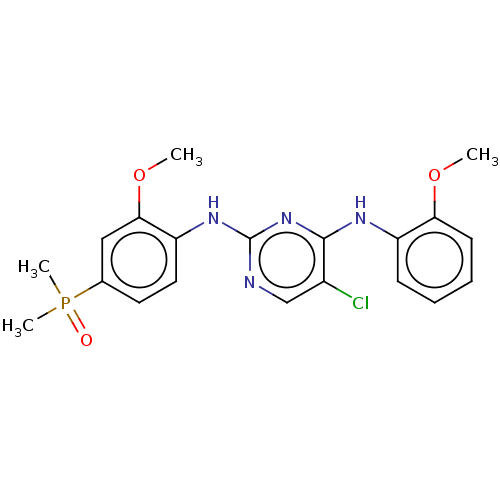
(CHEMBL3822982)Show SMILES COc1ccccc1Nc1nc(Nc2ccc(cc2OC)P(C)(C)=O)ncc1Cl Show InChI InChI=1S/C20H22ClN4O3P/c1-27-17-8-6-5-7-15(17)23-19-14(21)12-22-20(25-19)24-16-10-9-13(29(3,4)26)11-18(16)28-2/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185153

(CHEMBL3822499)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H38ClN7O2/c1-20(38)23-6-4-5-7-25(23)32-28-24(30)19-31-29(34-28)33-26-9-8-22(18-27(26)39-3)36-12-10-21(11-13-36)37-16-14-35(2)15-17-37/h4-9,18-21,38H,10-17H2,1-3H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185236
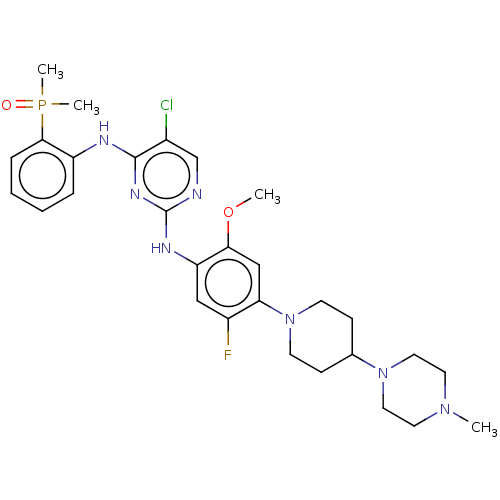
(CHEMBL3823078)Show SMILES COc1cc(N2CCC(CC2)N2CCN(C)CC2)c(F)cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C29H38ClFN7O2P/c1-36-13-15-37(16-14-36)20-9-11-38(12-10-20)25-18-26(40-2)24(17-22(25)31)34-29-32-19-21(30)28(35-29)33-23-7-5-6-8-27(23)41(3,4)39/h5-8,17-20H,9-16H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185285
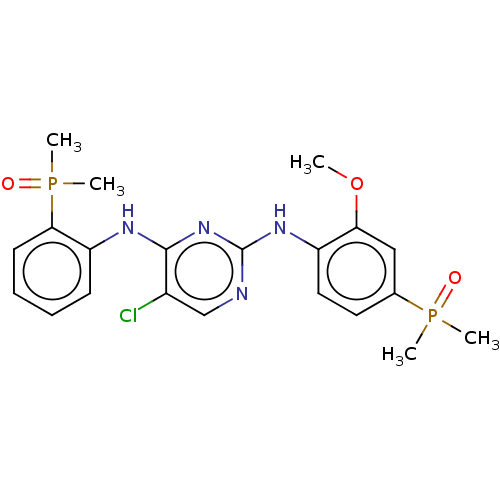
(CHEMBL3823165)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)P(C)(C)=O Show InChI InChI=1S/C21H25ClN4O3P2/c1-29-18-12-14(30(2,3)27)10-11-16(18)25-21-23-13-15(22)20(26-21)24-17-8-6-7-9-19(17)31(4,5)28/h6-13H,1-5H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185240

(CHEMBL3823198)Show SMILES COc1cc(ccc1Nc1ncc(OC)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H42N7O3P/c1-35-16-18-37(19-17-35)22-12-14-36(15-13-22)23-10-11-24(26(20-23)39-2)33-30-31-21-27(40-3)29(34-30)32-25-8-6-7-9-28(25)41(4,5)38/h6-11,20-22H,12-19H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185160
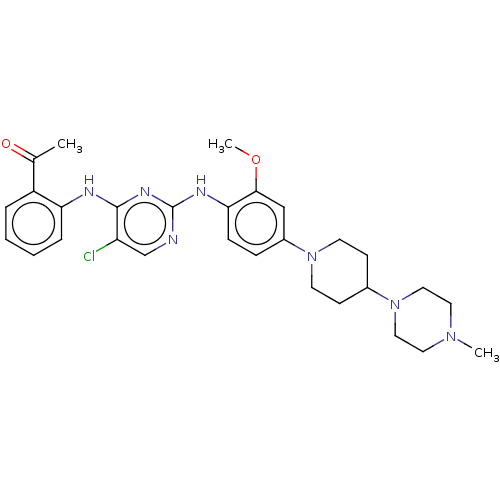
(CHEMBL3824301)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H36ClN7O2/c1-20(38)23-6-4-5-7-25(23)32-28-24(30)19-31-29(34-28)33-26-9-8-22(18-27(26)39-3)36-12-10-21(11-13-36)37-16-14-35(2)15-17-37/h4-9,18-19,21H,10-17H2,1-3H3,(H2,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185163

(CHEMBL3824246)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C28H35ClN8O2/c1-35-13-15-37(16-14-35)19-9-11-36(12-10-19)20-7-8-24(25(17-20)39-2)33-28-31-18-22(29)27(34-28)32-23-6-4-3-5-21(23)26(30)38/h3-8,17-19H,9-16H2,1-2H3,(H2,30,38)(H2,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185166
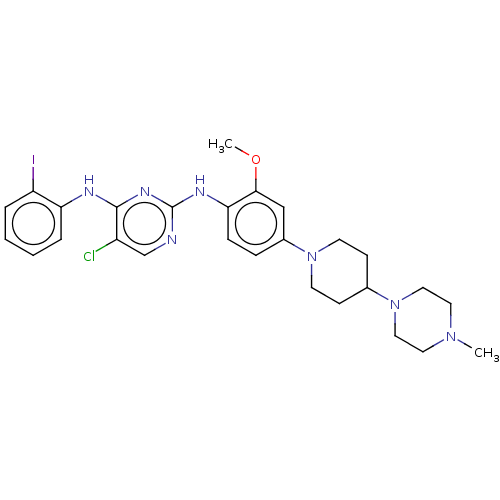
(CHEMBL3822664)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2I)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C27H33ClIN7O/c1-34-13-15-36(16-14-34)19-9-11-35(12-10-19)20-7-8-24(25(17-20)37-2)32-27-30-18-21(28)26(33-27)31-23-6-4-3-5-22(23)29/h3-8,17-19H,9-16H2,1-2H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data