

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296179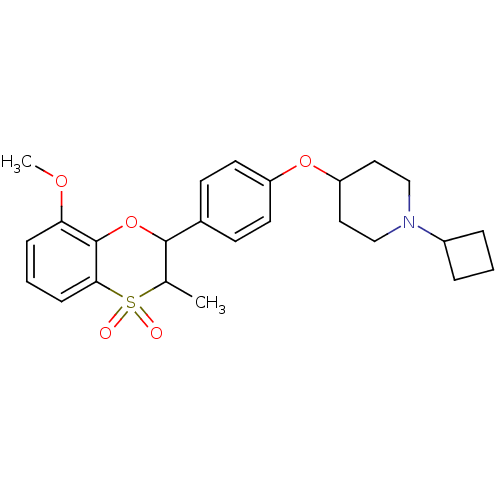 ((+/-)-1-Cyclobutyl-4-[4-(8-methoxy-3-methyl-4,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296178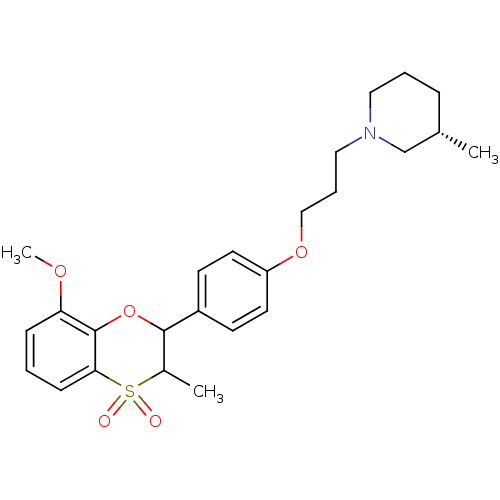 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296176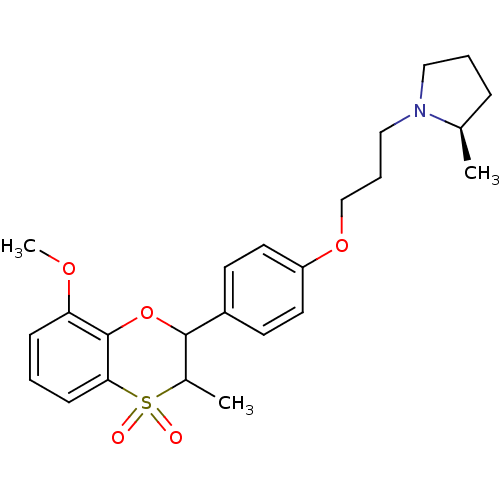 ((+/-)-(R)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296174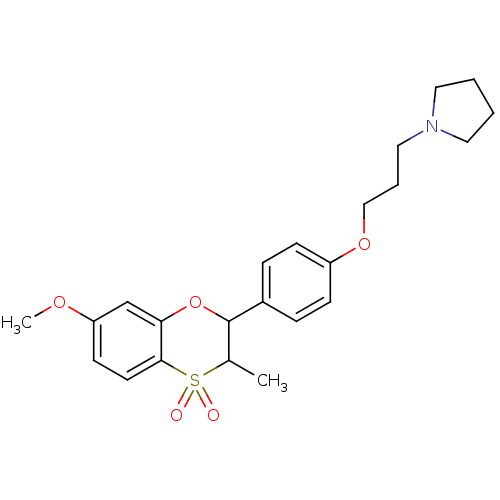 ((+/-)-1-{3-[4-(7-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296175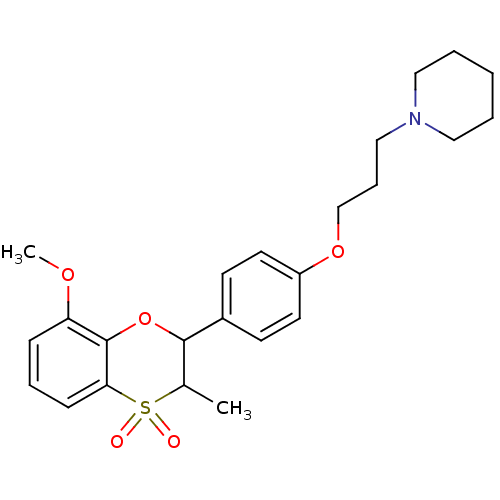 ((+/-)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296173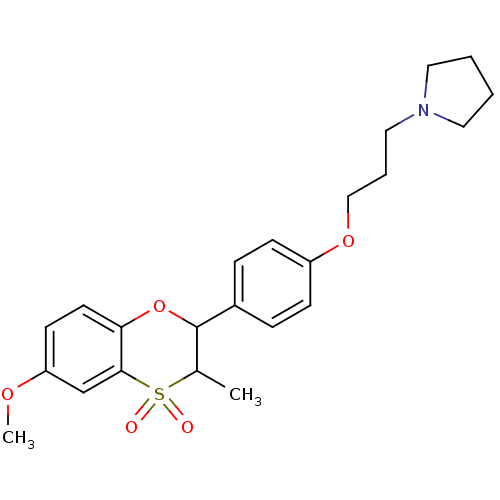 ((+/-)-1-{3-[4-(6-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296172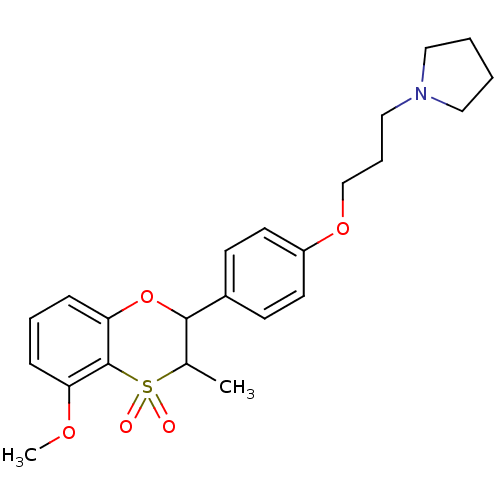 ((+/-)-1-{3-[4-(5-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296181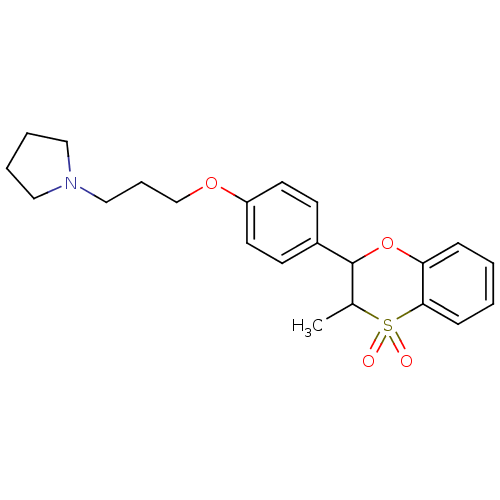 ((+/-)-1-{3-[4-(3-Methyl-4,4-dioxo-3,4-dihydro-2H-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296188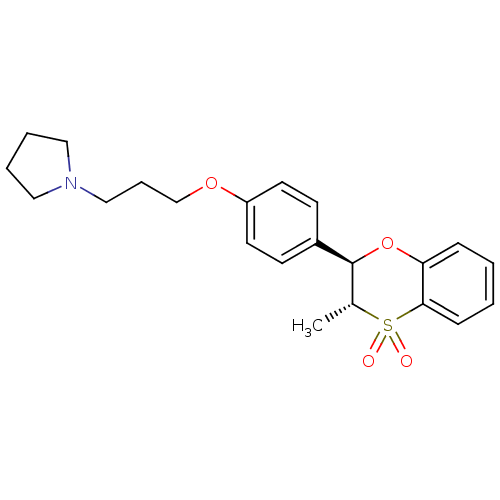 (CHEMBL558248 | trans-1-{3-[4-(3-Methyl-4,4-dioxo-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296180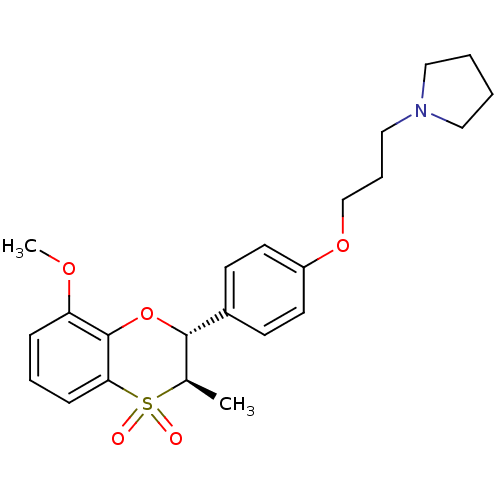 (1-{3-[4-((2R,3R)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.607 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296177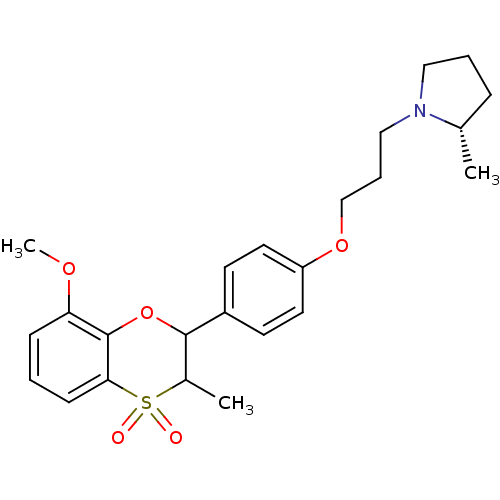 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296189 (1-{3-[4-(3-Methyl-4,4-dioxo-4H-4lambda*6*-benzo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296185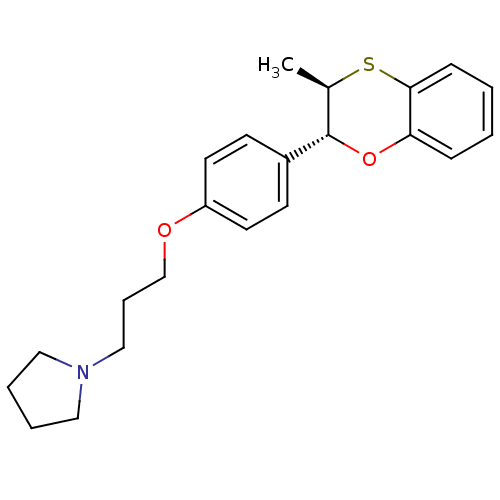 (CHEMBL550393 | trans-1-(3-(4-(3-methyl-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296184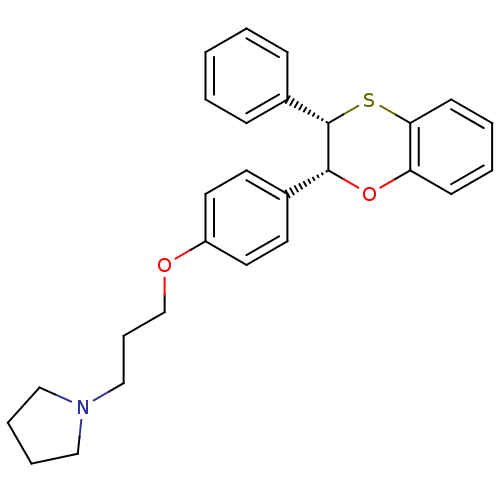 (CHEMBL564514 | cis-1-(3-(4-(3-phenyl-2,3-dihydrobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296187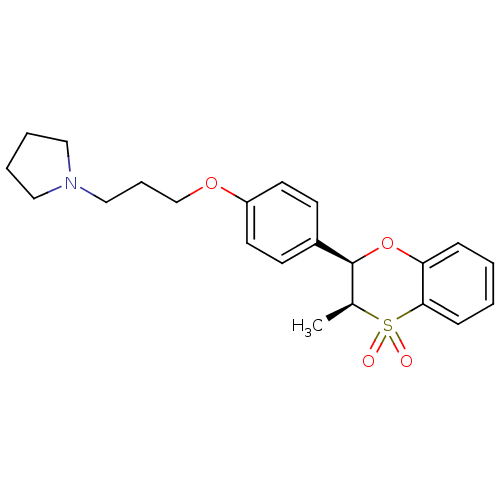 (CHEMBL549908 | cis-1-{3-[4-(3-Methyl-4,4-dioxo-3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296186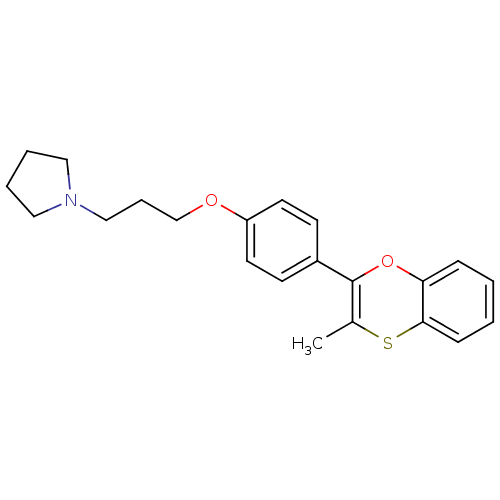 (1-(3-(4-(3-methylbenzo[b][1,4]oxathiin-2-yl)phenox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296182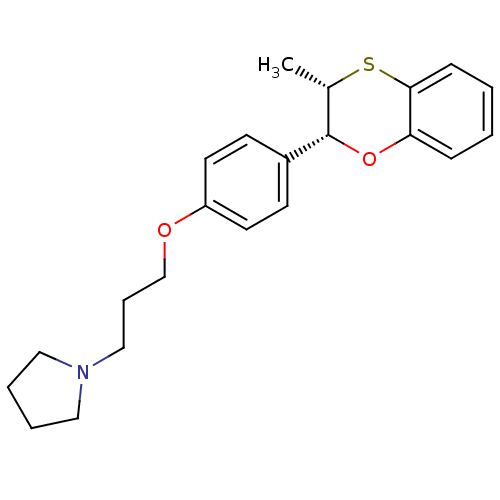 (CHEMBL557853 | cis-1-(3-(4-(3-methyl-2,3-dihydrobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296183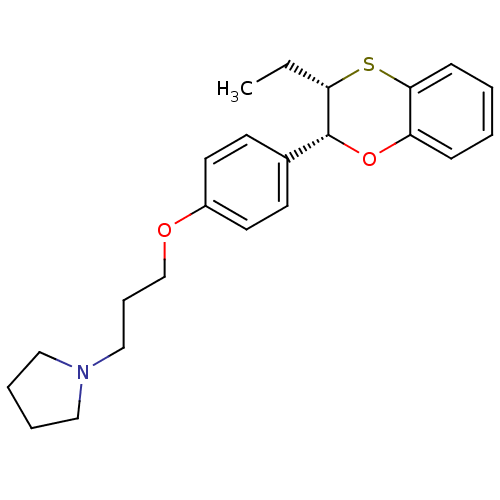 (CHEMBL549433 | cis-1-(3-(4-(3-ethyl-2,3-dihydroben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296182 (CHEMBL557853 | cis-1-(3-(4-(3-methyl-2,3-dihydrobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296186 (1-(3-(4-(3-methylbenzo[b][1,4]oxathiin-2-yl)phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296183 (CHEMBL549433 | cis-1-(3-(4-(3-ethyl-2,3-dihydroben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296184 (CHEMBL564514 | cis-1-(3-(4-(3-phenyl-2,3-dihydrobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296185 (CHEMBL550393 | trans-1-(3-(4-(3-methyl-2,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296187 (CHEMBL549908 | cis-1-{3-[4-(3-Methyl-4,4-dioxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296189 (1-{3-[4-(3-Methyl-4,4-dioxo-4H-4lambda*6*-benzo[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296173 ((+/-)-1-{3-[4-(6-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296181 ((+/-)-1-{3-[4-(3-Methyl-4,4-dioxo-3,4-dihydro-2H-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296188 (CHEMBL558248 | trans-1-{3-[4-(3-Methyl-4,4-dioxo-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296174 ((+/-)-1-{3-[4-(7-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296178 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296176 ((+/-)-(R)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296177 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296175 ((+/-)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296180 (1-{3-[4-((2R,3R)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296172 ((+/-)-1-{3-[4-(5-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human histamine H4 receptor | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human histamine H2 receptor | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of human histamine H1 receptor | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296179 ((+/-)-1-Cyclobutyl-4-[4-(8-methoxy-3-methyl-4,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [35S]MK499 binding to human ERG transfected in HEK293 cells by microscintillation counting | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||