 Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50047817
Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50047817 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine C-terminal truncated GRK5 (561 residues) assessed as decrease in phosphorylation of urea-washed bovine rod outer segments preinc... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 2
(Bos taurus) | BDBM50191311
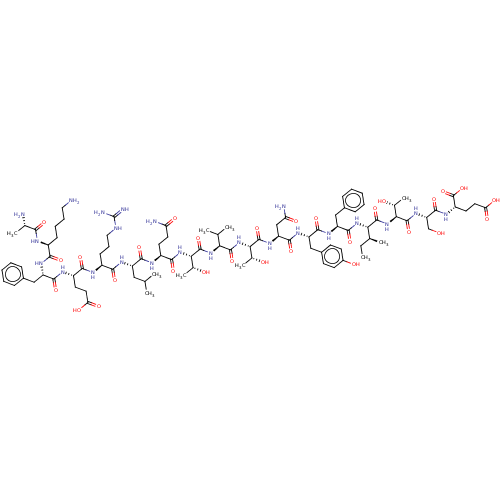
(CHEMBL3950887)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)N)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C93H143N23O29/c1-11-47(6)72(88(140)116-74(50(9)119)90(142)111-66(44-117)86(138)105-60(92(144)145)33-36-70(126)127)113-85(137)64(41-53-23-16-13-17-24-53)109-83(135)63(42-54-27-29-55(121)30-28-54)108-84(136)65(43-68(97)123)110-89(141)73(49(8)118)115-87(139)71(46(4)5)112-91(143)75(51(10)120)114-80(132)58(31-34-67(96)122)103-81(133)61(39-45(2)3)106-78(130)57(26-20-38-100-93(98)99)102-79(131)59(32-35-69(124)125)104-82(134)62(40-52-21-14-12-15-22-52)107-77(129)56(25-18-19-37-94)101-76(128)48(7)95/h12-17,21-24,27-30,45-51,56-66,71-75,117-121H,11,18-20,25-26,31-44,94-95H2,1-10H3,(H2,96,122)(H2,97,123)(H,101,128)(H,102,131)(H,103,133)(H,104,134)(H,105,138)(H,106,130)(H,107,129)(H,108,136)(H,109,135)(H,110,141)(H,111,142)(H,112,143)(H,113,137)(H,114,132)(H,115,139)(H,116,140)(H,124,125)(H,126,127)(H,144,145)(H4,98,99,100)/t47-,48-,49+,50+,51+,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,71-,72-,73-,74-,75-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant bovine GRK3 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50191311

(CHEMBL3950887)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)N)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C93H143N23O29/c1-11-47(6)72(88(140)116-74(50(9)119)90(142)111-66(44-117)86(138)105-60(92(144)145)33-36-70(126)127)113-85(137)64(41-53-23-16-13-17-24-53)109-83(135)63(42-54-27-29-55(121)30-28-54)108-84(136)65(43-68(97)123)110-89(141)73(49(8)118)115-87(139)71(46(4)5)112-91(143)75(51(10)120)114-80(132)58(31-34-67(96)122)103-81(133)61(39-45(2)3)106-78(130)57(26-20-38-100-93(98)99)102-79(131)59(32-35-69(124)125)104-82(134)62(40-52-21-14-12-15-22-52)107-77(129)56(25-18-19-37-94)101-76(128)48(7)95/h12-17,21-24,27-30,45-51,56-66,71-75,117-121H,11,18-20,25-26,31-44,94-95H2,1-10H3,(H2,96,122)(H2,97,123)(H,101,128)(H,102,131)(H,103,133)(H,104,134)(H,105,138)(H,106,130)(H,107,129)(H,108,136)(H,109,135)(H,110,141)(H,111,142)(H,112,143)(H,113,137)(H,114,132)(H,115,139)(H,116,140)(H,124,125)(H,126,127)(H,144,145)(H4,98,99,100)/t47-,48-,49+,50+,51+,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,71-,72-,73-,74-,75-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GRK5 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments i... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50191319
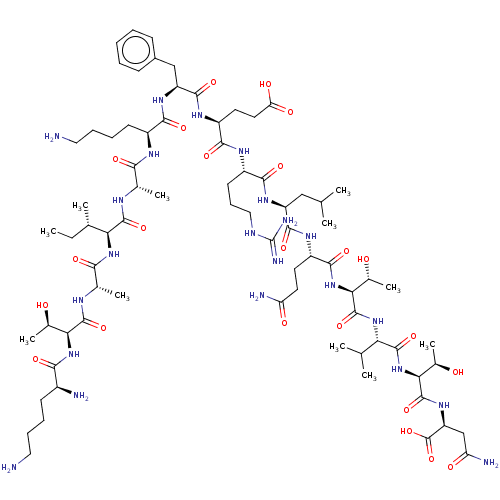
(CHEMBL3942164)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O |r| Show InChI InChI=1S/C76H130N22O23/c1-12-38(6)57(95-62(107)40(8)86-72(117)58(41(9)99)96-63(108)45(79)23-16-18-30-77)71(116)85-39(7)61(106)87-46(24-17-19-31-78)64(109)92-51(34-44-21-14-13-15-22-44)69(114)90-49(27-29-55(104)105)66(111)88-47(25-20-32-84-76(82)83)65(110)91-50(33-36(2)3)68(113)89-48(26-28-53(80)102)67(112)97-60(43(11)101)74(119)94-56(37(4)5)70(115)98-59(42(10)100)73(118)93-52(75(120)121)35-54(81)103/h13-15,21-22,36-43,45-52,56-60,99-101H,12,16-20,23-35,77-79H2,1-11H3,(H2,80,102)(H2,81,103)(H,85,116)(H,86,117)(H,87,106)(H,88,111)(H,89,113)(H,90,114)(H,91,110)(H,92,109)(H,93,118)(H,94,119)(H,95,107)(H,96,108)(H,97,112)(H,98,115)(H,104,105)(H,120,121)(H4,82,83,84)/t38-,39-,40-,41+,42+,43+,45-,46-,47-,48-,49-,50-,51-,52-,56-,57-,58-,59-,60-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GRK5 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments i... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50191313
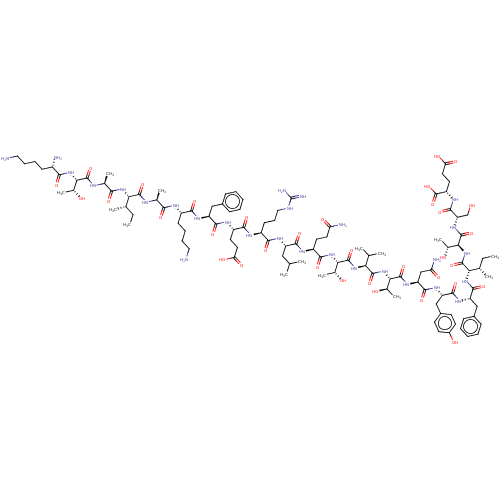
(CHEMBL3931500)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C112H178N28O34/c1-15-56(7)85(135-92(154)59(10)122-107(169)87(60(11)142)137-93(155)68(115)32-23-25-45-113)105(167)121-58(9)91(153)123-69(33-24-26-46-114)94(156)129-75(49-64-28-19-17-20-29-64)99(161)126-72(40-43-82(149)150)96(158)124-70(34-27-47-120-112(118)119)95(157)128-74(48-54(3)4)98(160)125-71(39-42-80(116)147)97(159)138-90(63(14)145)110(172)134-84(55(5)6)104(166)139-88(61(12)143)108(170)132-78(52-81(117)148)101(163)130-76(51-66-35-37-67(146)38-36-66)100(162)131-77(50-65-30-21-18-22-31-65)102(164)136-86(57(8)16-2)106(168)140-89(62(13)144)109(171)133-79(53-141)103(165)127-73(111(173)174)41-44-83(151)152/h17-22,28-31,35-38,54-63,68-79,84-90,141-146H,15-16,23-27,32-34,39-53,113-115H2,1-14H3,(H2,116,147)(H2,117,148)(H,121,167)(H,122,169)(H,123,153)(H,124,158)(H,125,160)(H,126,161)(H,127,165)(H,128,157)(H,129,156)(H,130,163)(H,131,162)(H,132,170)(H,133,171)(H,134,172)(H,135,154)(H,136,164)(H,137,155)(H,138,159)(H,139,166)(H,140,168)(H,149,150)(H,151,152)(H,173,174)(H4,118,119,120)/t56-,57-,58-,59-,60+,61+,62+,63+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GRK5 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments i... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine C-terminal truncated GRK1 (535 residues) assessed as decrease in phosphorylation of urea-washed bovine rod outer segments preinc... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 2
(Bos taurus) | BDBM50191313

(CHEMBL3931500)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C112H178N28O34/c1-15-56(7)85(135-92(154)59(10)122-107(169)87(60(11)142)137-93(155)68(115)32-23-25-45-113)105(167)121-58(9)91(153)123-69(33-24-26-46-114)94(156)129-75(49-64-28-19-17-20-29-64)99(161)126-72(40-43-82(149)150)96(158)124-70(34-27-47-120-112(118)119)95(157)128-74(48-54(3)4)98(160)125-71(39-42-80(116)147)97(159)138-90(63(14)145)110(172)134-84(55(5)6)104(166)139-88(61(12)143)108(170)132-78(52-81(117)148)101(163)130-76(51-66-35-37-67(146)38-36-66)100(162)131-77(50-65-30-21-18-22-31-65)102(164)136-86(57(8)16-2)106(168)140-89(62(13)144)109(171)133-79(53-141)103(165)127-73(111(173)174)41-44-83(151)152/h17-22,28-31,35-38,54-63,68-79,84-90,141-146H,15-16,23-27,32-34,39-53,113-115H2,1-14H3,(H2,116,147)(H2,117,148)(H,121,167)(H,122,169)(H,123,153)(H,124,158)(H,125,160)(H,126,161)(H,127,165)(H,128,157)(H,129,156)(H,130,163)(H,131,162)(H,132,170)(H,133,171)(H,134,172)(H,135,154)(H,136,164)(H,137,155)(H,138,159)(H,139,166)(H,140,168)(H,149,150)(H,151,152)(H,173,174)(H4,118,119,120)/t56-,57-,58-,59-,60+,61+,62+,63+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant bovine GRK3 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50191326
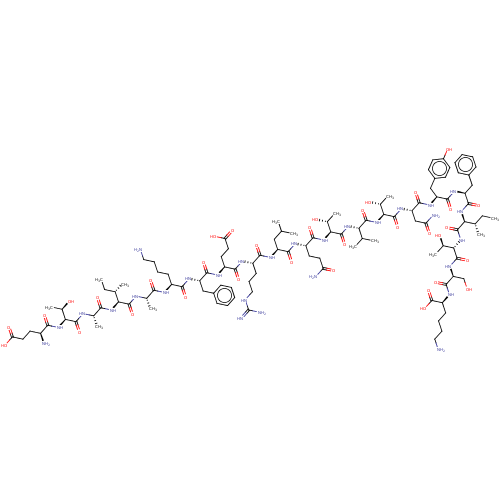
(CHEMBL3953837)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C112H178N28O34/c1-15-56(7)85(135-92(154)59(10)122-107(169)87(60(11)142)137-93(155)68(115)39-43-82(149)150)105(167)121-58(9)91(153)123-69(32-23-25-45-113)94(156)129-75(49-64-28-19-17-20-29-64)99(161)126-72(41-44-83(151)152)96(158)124-70(34-27-47-120-112(118)119)95(157)128-74(48-54(3)4)98(160)125-71(40-42-80(116)147)97(159)138-90(63(14)145)110(172)134-84(55(5)6)104(166)139-88(61(12)143)108(170)132-78(52-81(117)148)101(163)130-76(51-66-35-37-67(146)38-36-66)100(162)131-77(50-65-30-21-18-22-31-65)102(164)136-86(57(8)16-2)106(168)140-89(62(13)144)109(171)133-79(53-141)103(165)127-73(111(173)174)33-24-26-46-114/h17-22,28-31,35-38,54-63,68-79,84-90,141-146H,15-16,23-27,32-34,39-53,113-115H2,1-14H3,(H2,116,147)(H2,117,148)(H,121,167)(H,122,169)(H,123,153)(H,124,158)(H,125,160)(H,126,161)(H,127,165)(H,128,157)(H,129,156)(H,130,163)(H,131,162)(H,132,170)(H,133,171)(H,134,172)(H,135,154)(H,136,164)(H,137,155)(H,138,159)(H,139,166)(H,140,168)(H,149,150)(H,151,152)(H,173,174)(H4,118,119,120)/t56-,57-,58-,59-,60+,61+,62+,63+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GRK5 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments i... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50191310
(CHEMBL3971763)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)N)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C94H148N24O27/c1-11-49(6)73(89(140)118-75(52(9)121)91(142)113-68(46-119)87(138)107-62(93(144)145)28-19-21-39-96)115-86(137)66(43-55-25-16-13-17-26-55)111-84(135)65(44-56-30-32-57(123)33-31-56)110-85(136)67(45-70(99)125)112-90(141)74(51(8)120)117-88(139)72(48(4)5)114-92(143)76(53(10)122)116-81(132)60(34-36-69(98)124)105-82(133)63(41-47(2)3)108-79(130)59(29-22-40-102-94(100)101)104-80(131)61(35-37-71(126)127)106-83(134)64(42-54-23-14-12-15-24-54)109-78(129)58(27-18-20-38-95)103-77(128)50(7)97/h12-17,23-26,30-33,47-53,58-68,72-76,119-123H,11,18-22,27-29,34-46,95-97H2,1-10H3,(H2,98,124)(H2,99,125)(H,103,128)(H,104,131)(H,105,133)(H,106,134)(H,107,138)(H,108,130)(H,109,129)(H,110,136)(H,111,135)(H,112,141)(H,113,142)(H,114,143)(H,115,137)(H,116,132)(H,117,139)(H,118,140)(H,126,127)(H,144,145)(H4,100,101,102)/t49-,50-,51+,52+,53+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,72-,73-,74-,75-,76-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GRK5 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments i... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 2
(Bos taurus) | BDBM50191319

(CHEMBL3942164)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O |r| Show InChI InChI=1S/C76H130N22O23/c1-12-38(6)57(95-62(107)40(8)86-72(117)58(41(9)99)96-63(108)45(79)23-16-18-30-77)71(116)85-39(7)61(106)87-46(24-17-19-31-78)64(109)92-51(34-44-21-14-13-15-22-44)69(114)90-49(27-29-55(104)105)66(111)88-47(25-20-32-84-76(82)83)65(110)91-50(33-36(2)3)68(113)89-48(26-28-53(80)102)67(112)97-60(43(11)101)74(119)94-56(37(4)5)70(115)98-59(42(10)100)73(118)93-52(75(120)121)35-54(81)103/h13-15,21-22,36-43,45-52,56-60,99-101H,12,16-20,23-35,77-79H2,1-11H3,(H2,80,102)(H2,81,103)(H,85,116)(H,86,117)(H,87,106)(H,88,111)(H,89,113)(H,90,114)(H,91,110)(H,92,109)(H,93,118)(H,94,119)(H,95,107)(H,96,108)(H,97,112)(H,98,115)(H,104,105)(H,120,121)(H4,82,83,84)/t38-,39-,40-,41+,42+,43+,45-,46-,47-,48-,49-,50-,51-,52-,56-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant bovine GRK3 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Bos taurus) | BDBM50191320
(CHEMBL3945401)Show SMILES OS(=O)(=O)O[C@H]1[C@H](OS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@@H]1OS(O)(=O)=O |r| Show InChI InChI=1S/C6H12O24S6/c7-31(8,9)25-1-2(26-32(10,11)12)4(28-34(16,17)18)6(30-36(22,23)24)5(29-35(19,20)21)3(1)27-33(13,14)15/h1-6H,(H,7,8,9)(H,10,11,12)(H,13,14,15)(H,16,17,18)(H,19,20,21)(H,22,23,24)/t1-,2-,3-,4+,5-,6- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine cerebral cortex GRK2 assessed as decrease in rhodopsin phosphorylation by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 2
(Bos taurus) | BDBM50191326

(CHEMBL3953837)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C112H178N28O34/c1-15-56(7)85(135-92(154)59(10)122-107(169)87(60(11)142)137-93(155)68(115)39-43-82(149)150)105(167)121-58(9)91(153)123-69(32-23-25-45-113)94(156)129-75(49-64-28-19-17-20-29-64)99(161)126-72(41-44-83(151)152)96(158)124-70(34-27-47-120-112(118)119)95(157)128-74(48-54(3)4)98(160)125-71(40-42-80(116)147)97(159)138-90(63(14)145)110(172)134-84(55(5)6)104(166)139-88(61(12)143)108(170)132-78(52-81(117)148)101(163)130-76(51-66-35-37-67(146)38-36-66)100(162)131-77(50-65-30-21-18-22-31-65)102(164)136-86(57(8)16-2)106(168)140-89(62(13)144)109(171)133-79(53-141)103(165)127-73(111(173)174)33-24-26-46-114/h17-22,28-31,35-38,54-63,68-79,84-90,141-146H,15-16,23-27,32-34,39-53,113-115H2,1-14H3,(H2,116,147)(H2,117,148)(H,121,167)(H,122,169)(H,123,153)(H,124,158)(H,125,160)(H,126,161)(H,127,165)(H,128,157)(H,129,156)(H,130,163)(H,131,162)(H,132,170)(H,133,171)(H,134,172)(H,135,154)(H,136,164)(H,137,155)(H,138,159)(H,139,166)(H,140,168)(H,149,150)(H,151,152)(H,173,174)(H4,118,119,120)/t56-,57-,58-,59-,60+,61+,62+,63+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,84-,85-,86-,87-,88-,89-,90-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant bovine GRK3 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 2
(Bos taurus) | BDBM50191312
(CHEMBL3900757)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C113H183N29O32/c1-15-58(7)86(137-93(154)61(10)124-108(169)88(62(11)144)139-94(155)70(117)34-23-26-46-114)106(167)123-60(9)92(153)125-71(35-24-27-47-115)95(156)131-77(51-66-30-19-17-20-31-66)100(161)128-74(43-45-84(151)152)97(158)126-72(37-29-49-122-113(120)121)96(157)130-76(50-56(3)4)99(160)127-73(42-44-82(118)149)98(159)140-91(65(14)147)111(172)136-85(57(5)6)105(166)141-89(63(12)145)109(170)134-80(54-83(119)150)102(163)132-78(53-68-38-40-69(148)41-39-68)101(162)133-79(52-67-32-21-18-22-33-67)103(164)138-87(59(8)16-2)107(168)142-90(64(13)146)110(171)135-81(55-143)104(165)129-75(112(173)174)36-25-28-48-116/h17-22,30-33,38-41,56-65,70-81,85-91,143-148H,15-16,23-29,34-37,42-55,114-117H2,1-14H3,(H2,118,149)(H2,119,150)(H,123,167)(H,124,169)(H,125,153)(H,126,158)(H,127,160)(H,128,161)(H,129,165)(H,130,157)(H,131,156)(H,132,163)(H,133,162)(H,134,170)(H,135,171)(H,136,172)(H,137,154)(H,138,164)(H,139,155)(H,140,159)(H,141,166)(H,142,168)(H,151,152)(H,173,174)(H4,120,121,122)/t58-,59-,60-,61-,62+,63+,64+,65+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,85-,86-,87-,88-,89-,90-,91-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant bovine GRK3 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM14056
(N-(7-chloro-1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(...)Show SMILES CC1=C(C(CC(=O)N1)c1ccc(cc1)C(F)(F)F)C(=O)Nc1cc(Cl)c2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C21H16ClF3N4O2/c1-10-18(20(31)28-14-6-12-9-26-29-19(12)16(22)7-14)15(8-17(30)27-10)11-2-4-13(5-3-11)21(23,24)25/h2-7,9,15H,8H2,1H3,(H,26,29)(H,27,30)(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK5 assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Bos taurus) | BDBM50191324
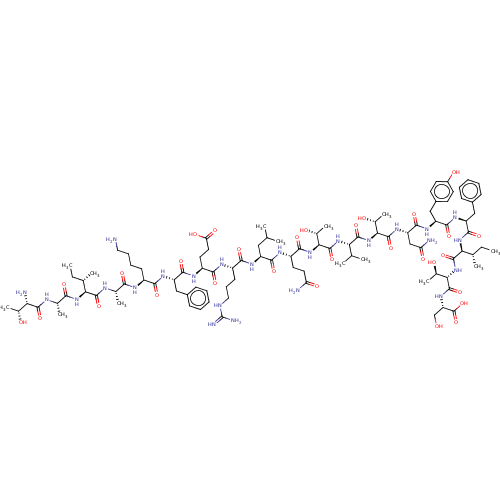
(CHEMBL3910956)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O |r| Show InChI InChI=1S/C101H159N25O30/c1-15-50(7)77(122-83(138)53(10)109-93(148)75(105)54(11)128)95(150)110-52(9)82(137)111-62(30-23-24-40-102)84(139)116-67(43-58-26-19-17-20-27-58)89(144)114-65(37-39-74(135)136)86(141)112-63(31-25-41-108-101(106)107)85(140)115-66(42-48(3)4)88(143)113-64(36-38-72(103)133)87(142)124-81(57(14)131)99(154)121-76(49(5)6)94(149)125-79(55(12)129)97(152)119-70(46-73(104)134)91(146)117-68(45-60-32-34-61(132)35-33-60)90(145)118-69(44-59-28-21-18-22-29-59)92(147)123-78(51(8)16-2)96(151)126-80(56(13)130)98(153)120-71(47-127)100(155)156/h17-22,26-29,32-35,48-57,62-71,75-81,127-132H,15-16,23-25,30-31,36-47,102,105H2,1-14H3,(H2,103,133)(H2,104,134)(H,109,148)(H,110,150)(H,111,137)(H,112,141)(H,113,143)(H,114,144)(H,115,140)(H,116,139)(H,117,146)(H,118,145)(H,119,152)(H,120,153)(H,121,154)(H,122,138)(H,123,147)(H,124,142)(H,125,149)(H,126,151)(H,135,136)(H,155,156)(H4,106,107,108)/t50-,51-,52-,53-,54+,55+,56+,57+,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,75-,76-,77-,78-,79-,80-,81-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine cerebral cortex GRK2 assessed as decrease in phosphorylation of beta-adrenoceptor in presence of [gamma-32P]-ATP by SDS-PAGE ana... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 2
(Bos taurus) | BDBM50191310

(CHEMBL3971763)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)N)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C94H148N24O27/c1-11-49(6)73(89(140)118-75(52(9)121)91(142)113-68(46-119)87(138)107-62(93(144)145)28-19-21-39-96)115-86(137)66(43-55-25-16-13-17-26-55)111-84(135)65(44-56-30-32-57(123)33-31-56)110-85(136)67(45-70(99)125)112-90(141)74(51(8)120)117-88(139)72(48(4)5)114-92(143)76(53(10)122)116-81(132)60(34-36-69(98)124)105-82(133)63(41-47(2)3)108-79(130)59(29-22-40-102-94(100)101)104-80(131)61(35-37-71(126)127)106-83(134)64(42-54-23-14-12-15-24-54)109-78(129)58(27-18-20-38-95)103-77(128)50(7)97/h12-17,23-26,30-33,47-53,58-68,72-76,119-123H,11,18-22,27-29,34-46,95-97H2,1-10H3,(H2,98,124)(H2,99,125)(H,103,128)(H,104,131)(H,105,133)(H,106,134)(H,107,138)(H,108,130)(H,109,129)(H,110,136)(H,111,135)(H,112,141)(H,113,142)(H,114,143)(H,115,137)(H,116,132)(H,117,139)(H,118,140)(H,126,127)(H,144,145)(H4,100,101,102)/t49-,50-,51+,52+,53+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,72-,73-,74-,75-,76-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant bovine GRK3 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50191312

(CHEMBL3900757)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C113H183N29O32/c1-15-58(7)86(137-93(154)61(10)124-108(169)88(62(11)144)139-94(155)70(117)34-23-26-46-114)106(167)123-60(9)92(153)125-71(35-24-27-47-115)95(156)131-77(51-66-30-19-17-20-31-66)100(161)128-74(43-45-84(151)152)97(158)126-72(37-29-49-122-113(120)121)96(157)130-76(50-56(3)4)99(160)127-73(42-44-82(118)149)98(159)140-91(65(14)147)111(172)136-85(57(5)6)105(166)141-89(63(12)145)109(170)134-80(54-83(119)150)102(163)132-78(53-68-38-40-69(148)41-39-68)101(162)133-79(52-67-32-21-18-22-33-67)103(164)138-87(59(8)16-2)107(168)142-90(64(13)146)110(171)135-81(55-143)104(165)129-75(112(173)174)36-25-28-48-116/h17-22,30-33,38-41,56-65,70-81,85-91,143-148H,15-16,23-29,34-37,42-55,114-117H2,1-14H3,(H2,118,149)(H2,119,150)(H,123,167)(H,124,169)(H,125,153)(H,126,158)(H,127,160)(H,128,161)(H,129,165)(H,130,157)(H,131,156)(H,132,163)(H,133,162)(H,134,170)(H,135,171)(H,136,172)(H,137,154)(H,138,164)(H,139,155)(H,140,159)(H,141,166)(H,142,168)(H,151,152)(H,173,174)(H4,120,121,122)/t58-,59-,60-,61-,62+,63+,64+,65+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,85-,86-,87-,88-,89-,90-,91-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GRK5 expressed in Sf9 insect cells assessed as decrease in phosphorylation of urea-washed bovine rod outer segments i... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Bos taurus) | BDBM50191322
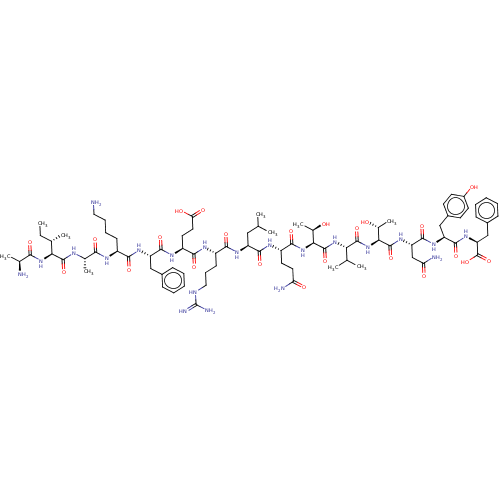
(CHEMBL3928291)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C84H129N21O23/c1-11-44(6)66(103-69(113)45(7)86)80(124)92-46(8)70(114)93-53(25-18-19-35-85)71(115)98-58(38-49-21-14-12-15-22-49)76(120)96-56(32-34-64(111)112)73(117)94-54(26-20-36-91-84(89)90)72(116)97-57(37-42(2)3)75(119)95-55(31-33-62(87)109)74(118)104-68(48(10)107)82(126)102-65(43(4)5)79(123)105-67(47(9)106)81(125)100-60(41-63(88)110)78(122)99-59(39-51-27-29-52(108)30-28-51)77(121)101-61(83(127)128)40-50-23-16-13-17-24-50/h12-17,21-24,27-30,42-48,53-61,65-68,106-108H,11,18-20,25-26,31-41,85-86H2,1-10H3,(H2,87,109)(H2,88,110)(H,92,124)(H,93,114)(H,94,117)(H,95,119)(H,96,120)(H,97,116)(H,98,115)(H,99,122)(H,100,125)(H,101,121)(H,102,126)(H,103,113)(H,104,118)(H,105,123)(H,111,112)(H,127,128)(H4,89,90,91)/t44-,45-,46-,47+,48+,53-,54-,55-,56-,57-,58-,59-,60-,61-,65-,66-,67-,68-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine cerebral cortex GRK2 assessed as decrease in phosphorylation of beta-adrenoceptor in presence of [gamma-32P]-ATP by SDS-PAGE ana... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM14052
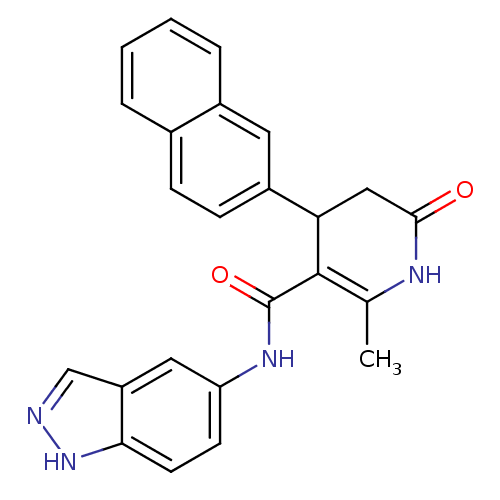
(N-(1H-indazol-5-yl)-2-methyl-4-(naphthalen-2-yl)-6...)Show SMILES CC1=C(C(CC(=O)N1)c1ccc2ccccc2c1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C24H20N4O2/c1-14-23(24(30)27-19-8-9-21-18(11-19)13-25-28-21)20(12-22(29)26-14)17-7-6-15-4-2-3-5-16(15)10-17/h2-11,13,20H,12H2,1H3,(H,25,28)(H,26,29)(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK5 assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM14051
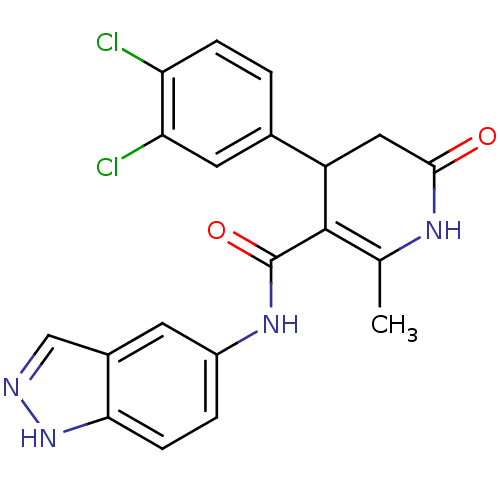
(4-(3,4-Dichlorophenyl)-N-1H-indazol-5-yl-2-methyl-...)Show SMILES CC1=C(C(CC(=O)N1)c1ccc(Cl)c(Cl)c1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C20H16Cl2N4O2/c1-10-19(20(28)25-13-3-5-17-12(6-13)9-23-26-17)14(8-18(27)24-10)11-2-4-15(21)16(22)7-11/h2-7,9,14H,8H2,1H3,(H,23,26)(H,24,27)(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK5 assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM50173320
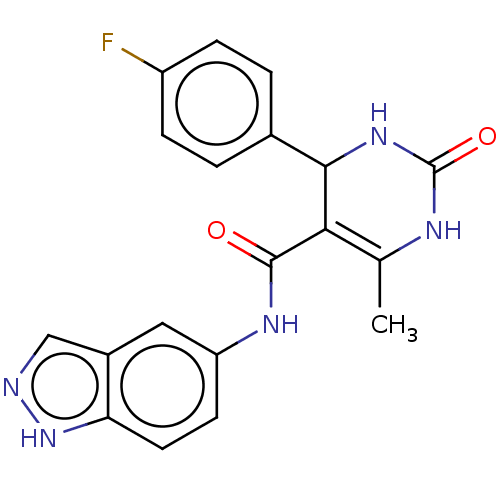
(GSK-180736A | GSK180736A | US10023564, Compound GS...)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(F)cc1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C19H16FN5O2/c1-10-16(17(24-19(27)22-10)11-2-4-13(20)5-3-11)18(26)23-14-6-7-15-12(8-14)9-21-25-15/h2-9,17H,1H3,(H,21,25)(H,23,26)(H2,22,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK5 assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM50331515
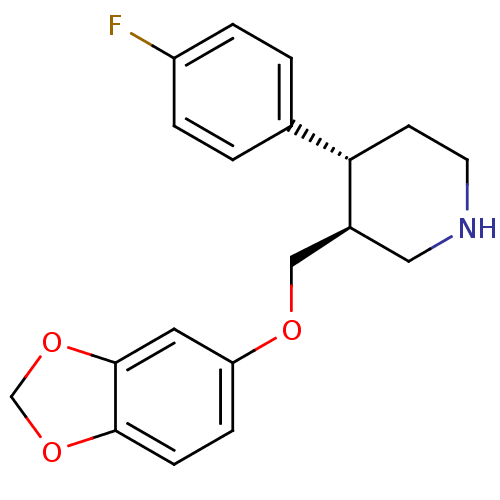
(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal hexahistidine tagged bovine GRK5 (561 residues) assessed as decrease in phosphorylation of tubulin preincubated for 30 mins ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM50331515

(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK5 assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM50331515

(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal hexahistidine tagged bovine GRK1 (535 residues) assessed as decrease in phosphorylation of tubulin preincubated for 30 mins ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50331515

(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length human N-terminal GST tagged PKCalpha expressed in Baculovirus infected Sf9 insect cells using KRREILSRRPSYR as ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM50331515

(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal hexahistidine tagged bovine GRK5 (561 residues) assessed as decrease in phosphorylation of light-activated bovine rod outer ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM50331515

(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal hexahistidine tagged bovine GRK1 (535 residues) assessed as decrease in phosphorylation of light-activated bovine rod outer ... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM50331515

(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 3.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 5
(Bos taurus) | BDBM50191325
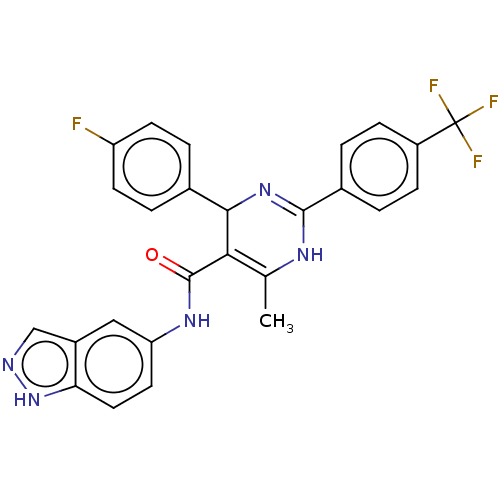
(CHEMBL3918278)Show SMILES CC1=C(C(N=C(N1)c1ccc(cc1)C(F)(F)F)c1ccc(F)cc1)C(=O)Nc1ccc2[nH]ncc2c1 |c:4,t:1| Show InChI InChI=1S/C26H19F4N5O/c1-14-22(25(36)33-20-10-11-21-17(12-20)13-31-35-21)23(15-4-8-19(27)9-5-15)34-24(32-14)16-2-6-18(7-3-16)26(28,29)30/h2-13,23H,1H3,(H,31,35)(H,32,34)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK5 assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM14052

(N-(1H-indazol-5-yl)-2-methyl-4-(naphthalen-2-yl)-6...)Show SMILES CC1=C(C(CC(=O)N1)c1ccc2ccccc2c1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C24H20N4O2/c1-14-23(24(30)27-19-8-9-21-18(11-19)13-25-28-21)20(12-22(29)26-14)17-7-6-15-4-2-3-5-16(15)10-17/h2-11,13,20H,12H2,1H3,(H,25,28)(H,26,29)(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM50173320

(GSK-180736A | GSK180736A | US10023564, Compound GS...)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(F)cc1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C19H16FN5O2/c1-10-16(17(24-19(27)22-10)11-2-4-13(20)5-3-11)18(26)23-14-6-7-15-12(8-14)9-21-25-15/h2-9,17H,1H3,(H,21,25)(H,23,26)(H2,22,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM14056

(N-(7-chloro-1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(...)Show SMILES CC1=C(C(CC(=O)N1)c1ccc(cc1)C(F)(F)F)C(=O)Nc1cc(Cl)c2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C21H16ClF3N4O2/c1-10-18(20(31)28-14-6-12-9-26-29-19(12)16(22)7-14)15(8-17(30)27-10)11-2-4-13(5-3-11)21(23,24)25/h2-7,9,15H,8H2,1H3,(H,26,29)(H,27,30)(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM50191325

(CHEMBL3918278)Show SMILES CC1=C(C(N=C(N1)c1ccc(cc1)C(F)(F)F)c1ccc(F)cc1)C(=O)Nc1ccc2[nH]ncc2c1 |c:4,t:1| Show InChI InChI=1S/C26H19F4N5O/c1-14-22(25(36)33-20-10-11-21-17(12-20)13-31-35-21)23(15-4-8-19(27)9-5-15)34-24(32-14)16-2-6-18(7-3-16)26(28,29)30/h2-13,23H,1H3,(H,31,35)(H,32,34)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK1
(Bos taurus) | BDBM14051

(4-(3,4-Dichlorophenyl)-N-1H-indazol-5-yl-2-methyl-...)Show SMILES CC1=C(C(CC(=O)N1)c1ccc(Cl)c(Cl)c1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| Show InChI InChI=1S/C20H16Cl2N4O2/c1-10-19(20(28)25-13-3-5-17-12(6-13)9-23-26-17)14(8-18(27)24-10)11-2-4-15(21)16(22)7-11/h2-7,9,14H,8H2,1H3,(H,23,26)(H,24,27)(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Bos taurus) | BDBM50191315
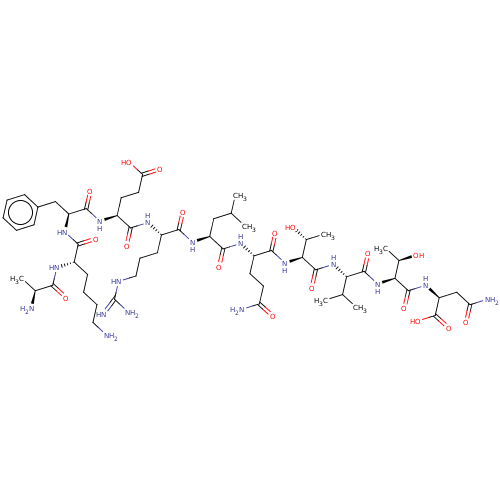
(CHEMBL3900347)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O |r| Show InChI InChI=1S/C57H95N17O18/c1-27(2)24-37(51(86)67-35(18-20-40(60)77)50(85)73-45(31(7)76)55(90)72-43(28(3)4)53(88)74-44(30(6)75)54(89)71-39(56(91)92)26-41(61)78)69-48(83)34(17-13-23-64-57(62)63)66-49(84)36(19-21-42(79)80)68-52(87)38(25-32-14-9-8-10-15-32)70-47(82)33(16-11-12-22-58)65-46(81)29(5)59/h8-10,14-15,27-31,33-39,43-45,75-76H,11-13,16-26,58-59H2,1-7H3,(H2,60,77)(H2,61,78)(H,65,81)(H,66,84)(H,67,86)(H,68,87)(H,69,83)(H,70,82)(H,71,89)(H,72,90)(H,73,85)(H,74,88)(H,79,80)(H,91,92)(H4,62,63,64)/t29-,30+,31+,33-,34-,35-,36-,37-,38-,39-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine cerebral cortex GRK2 assessed as decrease in phosphorylation of beta-adrenoceptor in presence of [gamma-32P]-ATP by SDS-PAGE ana... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Bos taurus) | BDBM50191309
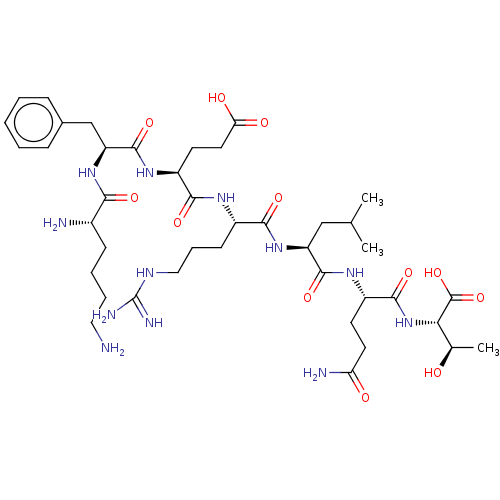
(CHEMBL3919384)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C41H68N12O12/c1-22(2)20-29(38(62)49-27(14-16-31(44)55)37(61)53-33(23(3)54)40(64)65)52-35(59)26(13-9-19-47-41(45)46)48-36(60)28(15-17-32(56)57)50-39(63)30(21-24-10-5-4-6-11-24)51-34(58)25(43)12-7-8-18-42/h4-6,10-11,22-23,25-30,33,54H,7-9,12-21,42-43H2,1-3H3,(H2,44,55)(H,48,60)(H,49,62)(H,50,63)(H,51,58)(H,52,59)(H,53,61)(H,56,57)(H,64,65)(H4,45,46,47)/t23-,25+,26+,27+,28+,29+,30+,33+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine cerebral cortex GRK2 assessed as decrease in phosphorylation of beta-adrenoceptor in presence of [gamma-32P]-ATP by SDS-PAGE ana... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Bos taurus) | BDBM50191323
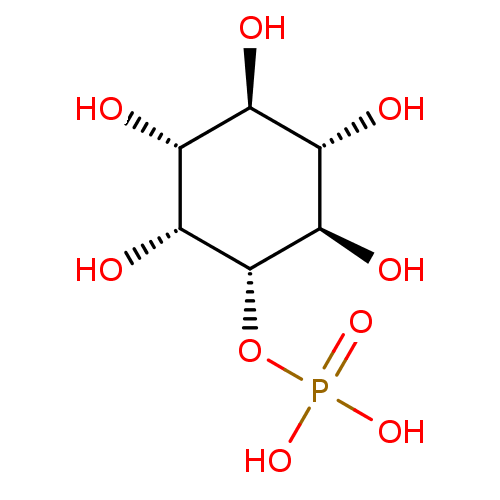
(CHEMBL3959608)Show SMILES O[C@H]1[C@H](O)[C@@H](O)[C@H](OP(O)(O)=O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H13O9P/c7-1-2(8)4(10)6(5(11)3(1)9)15-16(12,13)14/h1-11H,(H2,12,13,14)/t1-,2-,3+,4-,5-,6-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine cerebral cortex GRK2 assessed as decrease in rhodopsin phosphorylation by SDS-PAGE analysis |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant N-terminal GST-tagged PKGalpha expressed in Baculovirus infected insect Sf9 cells using RKRSRAE as substrate in... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Binding affinity to PKCalpha (unknown origin) using Lys-Arg-Thr-Leu-Arg-Arg as substrate after 8 mins in presence of [gamma-32P]ATP by liquid scintil... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Binding affinity to PKAalpha (unknown origin) using Lys-Arg-Thr-Leu-Arg-Arg as substrate after 8 mins in presence of [gamma-32P]ATP by liquid scintil... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM3149

(2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...)Show SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCCNC[C@H]1NC(=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C28H26N2O10/c31-16-8-6-14(7-9-16)26(36)30-18-13-29-10-2-5-22(18)40-28(39)15-11-20(33)24(21(34)12-15)25(35)23-17(27(37)38)3-1-4-19(23)32/h1,3-4,6-9,11-12,18,22,29,31-34H,2,5,10,13H2,(H,30,36)(H,37,38)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Binding affinity to PKCbeta2 (unknown origin) using Lys-Arg-Thr-Leu-Arg-Arg as substrate after 8 mins in presence of [gamma-32P]ATP by liquid scintil... |
J Med Chem 59: 9277-9294 (2016)
BindingDB Entry DOI: 10.7270/Q2348NB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data











































