

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196094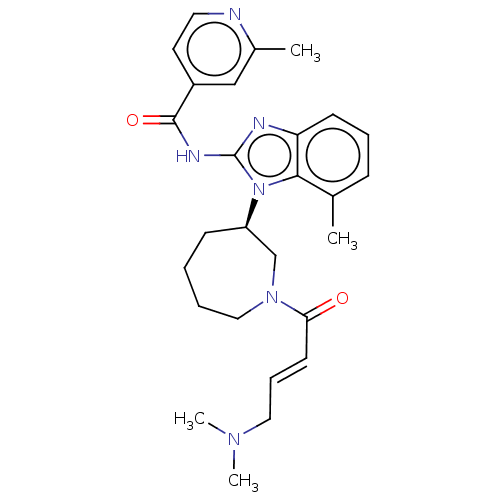 (CHEMBL3960167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196093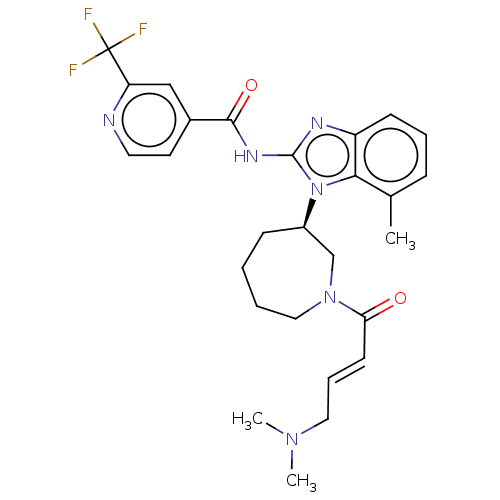 (CHEMBL3939913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50160870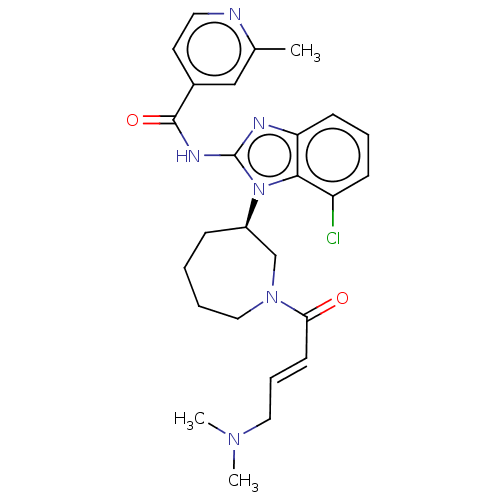 (CHEMBL3787344 | WO2022090481, Example nazartinib) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196095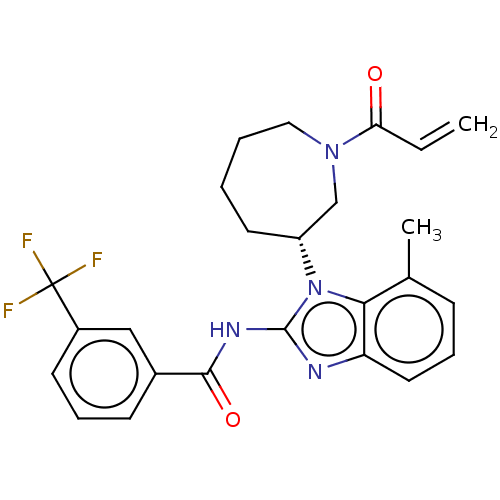 (CHEMBL3972316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM149404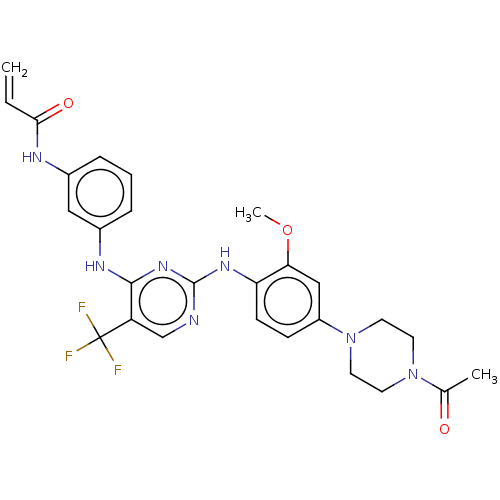 (AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196096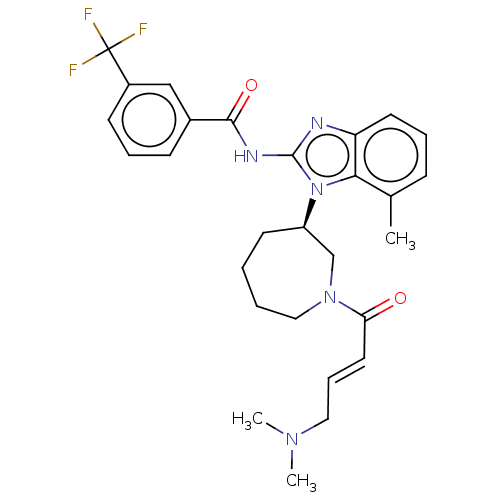 (CHEMBL3951434) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50196095 (CHEMBL3972316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50196095 (CHEMBL3972316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196101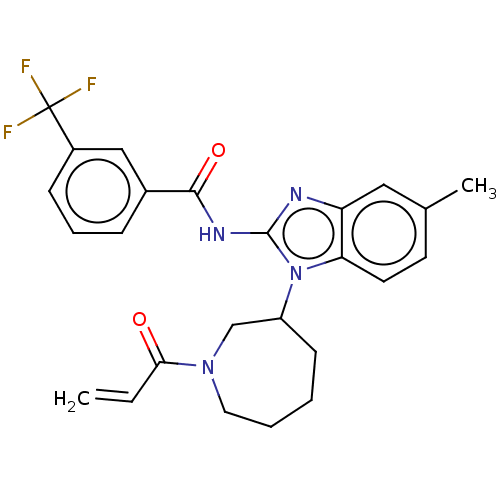 (CHEMBL3901943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196237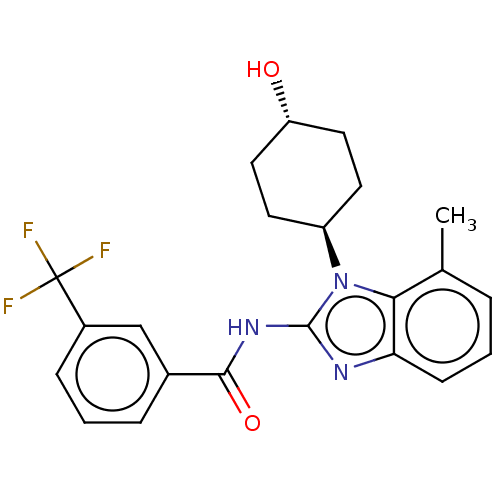 (CHEMBL3937373) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50196093 (CHEMBL3939913) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50160870 (CHEMBL3787344 | WO2022090481, Example nazartinib) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196103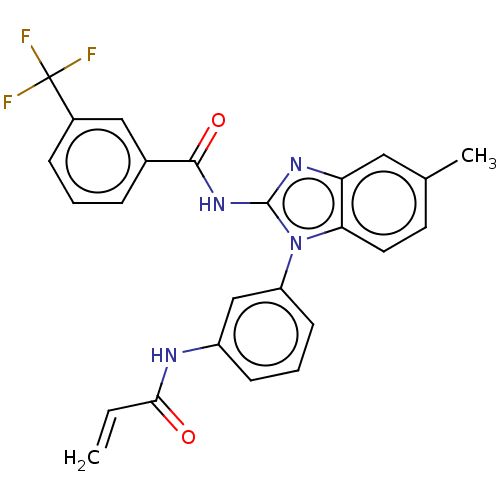 (CHEMBL3959632) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196102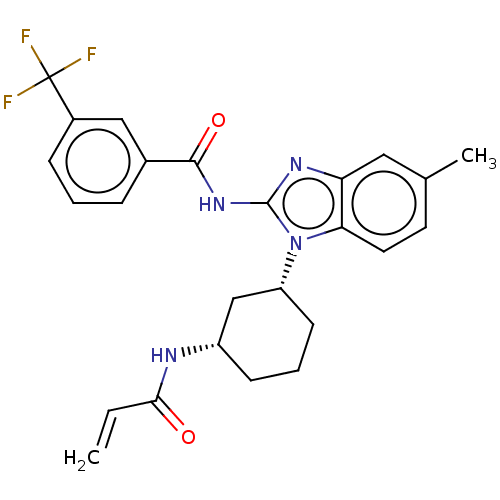 (CHEMBL3953221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type human EGFR phosphorylation expressed in mouse NIH/3T3 cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196099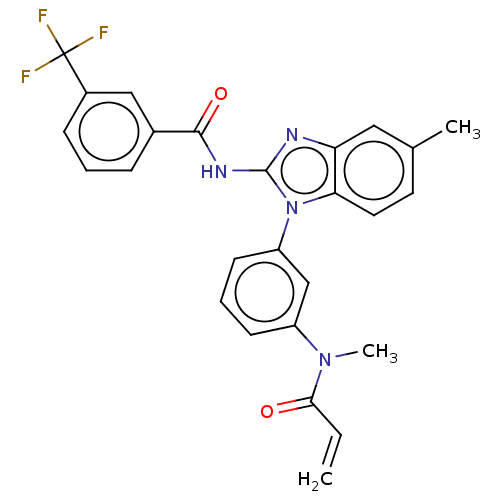 (CHEMBL3961202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196098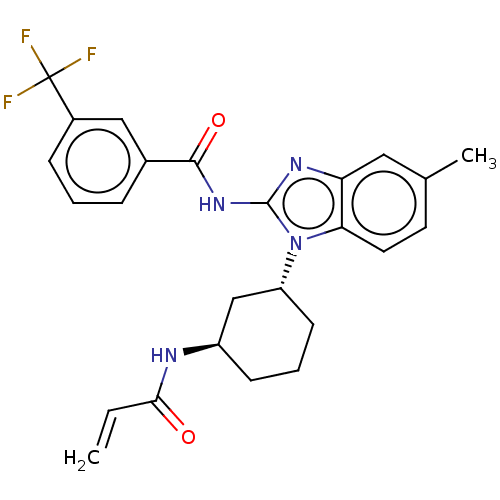 (CHEMBL3973973) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type human EGFR phosphorylation expressed in mouse NIH/3T3 cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196097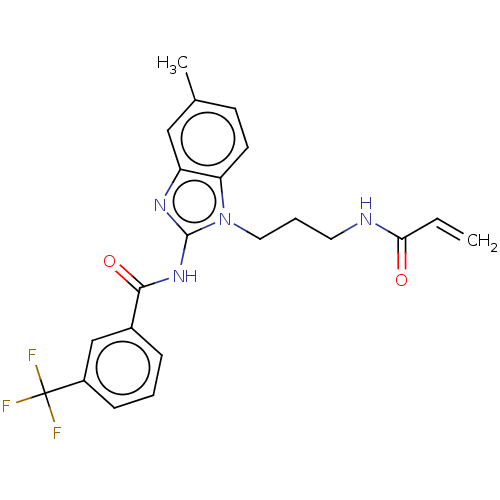 (CHEMBL3966111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196100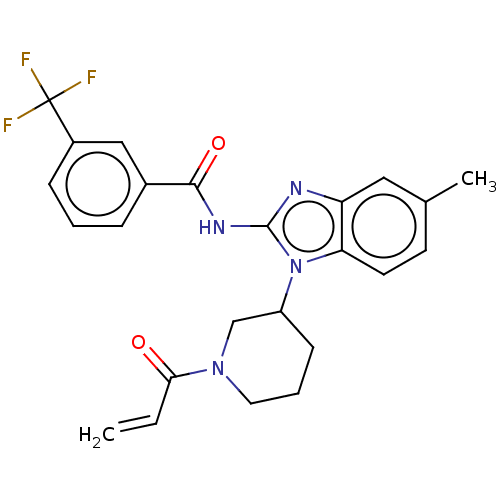 (CHEMBL3953921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196144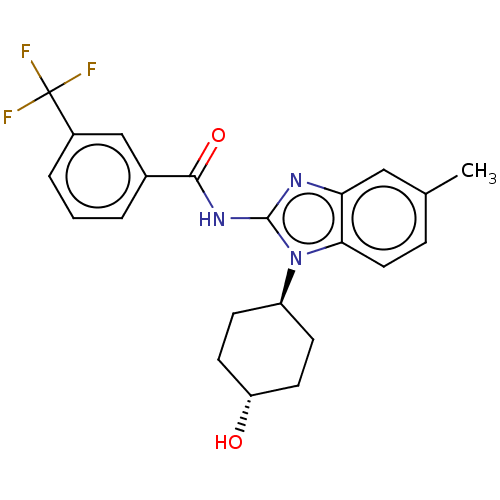 (CHEMBL3946259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50196096 (CHEMBL3951434) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50196096 (CHEMBL3951434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50196096 (CHEMBL3951434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50160870 (CHEMBL3787344 | WO2022090481, Example nazartinib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50160870 (CHEMBL3787344 | WO2022090481, Example nazartinib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50196094 (CHEMBL3960167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50196093 (CHEMBL3939913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50196095 (CHEMBL3972316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50196093 (CHEMBL3939913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50196094 (CHEMBL3960167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50196094 (CHEMBL3960167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196104 (CHEMBL3965075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196105 (CHEMBL3954370) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196238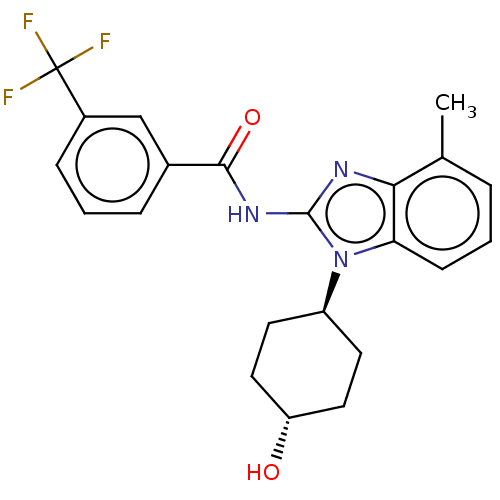 (CHEMBL3918367) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196236 (CHEMBL3909438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo... | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||