 Found 84 hits Enz. Inhib. hit(s) with all data for entry = 50048191
Found 84 hits Enz. Inhib. hit(s) with all data for entry = 50048191 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant human DAT expressed in CHO-S cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant human DAT expressed in CHO-S cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Paroxetine from recombinant human SERT expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Paroxetine from recombinant human SERT expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H] GR-65630 from recombinant human 5-HT3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H] GR-65630 from recombinant human 5-HT3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human Cav1.2 by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of ghrelin receptor (unknown origin) by radioligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207517
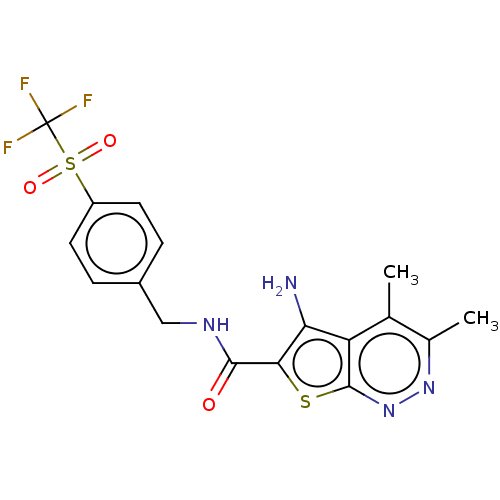
(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207515
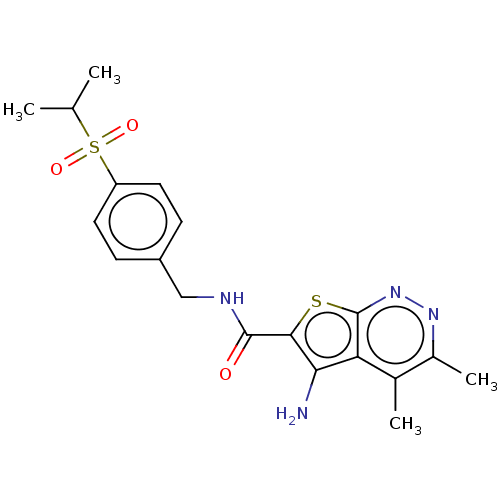
(CHEMBL3966208)Show SMILES CC(C)S(=O)(=O)c1ccc(CNC(=O)c2sc3nnc(C)c(C)c3c2N)cc1 Show InChI InChI=1S/C19H22N4O3S2/c1-10(2)28(25,26)14-7-5-13(6-8-14)9-21-18(24)17-16(20)15-11(3)12(4)22-23-19(15)27-17/h5-8,10H,9,20H2,1-4H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylscopolamine from human M1 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human M5 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M2 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M5 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylscopolamine from human M3 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylscopolamine from human M2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207518
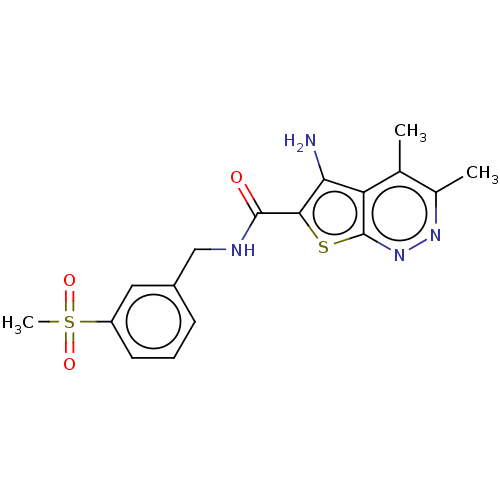
(CHEMBL3894866)Show SMILES Cc1nnc2sc(C(=O)NCc3cccc(c3)S(C)(=O)=O)c(N)c2c1C Show InChI InChI=1S/C17H18N4O3S2/c1-9-10(2)20-21-17-13(9)14(18)15(25-17)16(22)19-8-11-5-4-6-12(7-11)26(3,23)24/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 417 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50207519
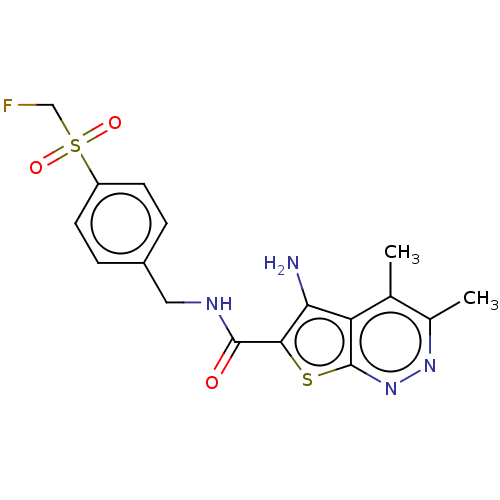
(CHEMBL3905112)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)CF)c(N)c2c1C Show InChI InChI=1S/C17H17FN4O3S2/c1-9-10(2)21-22-17-13(9)14(19)15(26-17)16(23)20-7-11-3-5-12(6-4-11)27(24,25)8-18/h3-6H,7-8,19H2,1-2H3,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M5 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50207520
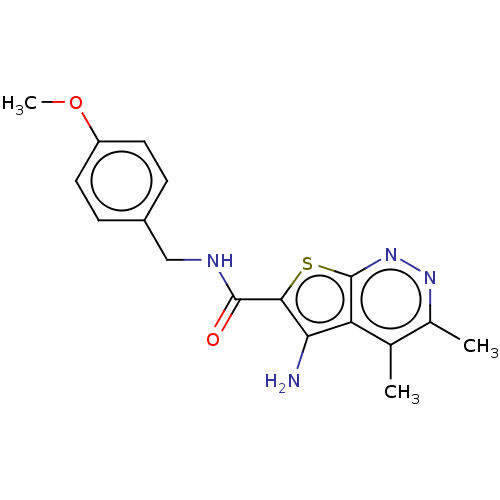
(CHEMBL3933538)Show InChI InChI=1S/C17H18N4O2S/c1-9-10(2)20-21-17-13(9)14(18)15(24-17)16(22)19-8-11-4-6-12(23-3)7-5-11/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human M4 receptor |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207519

(CHEMBL3905112)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)CF)c(N)c2c1C Show InChI InChI=1S/C17H17FN4O3S2/c1-9-10(2)21-22-17-13(9)14(19)15(26-17)16(23)20-7-11-3-5-12(6-4-11)27(24,25)8-18/h3-6H,7-8,19H2,1-2H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207521
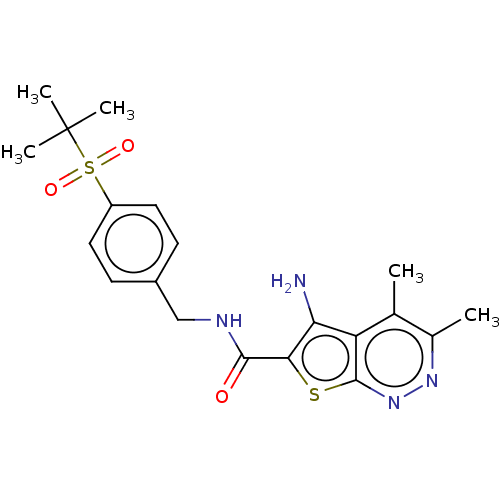
(CHEMBL3955059)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(C)(C)C)c(N)c2c1C Show InChI InChI=1S/C20H24N4O3S2/c1-11-12(2)23-24-19-15(11)16(21)17(28-19)18(25)22-10-13-6-8-14(9-7-13)29(26,27)20(3,4)5/h6-9H,10,21H2,1-5H3,(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207522
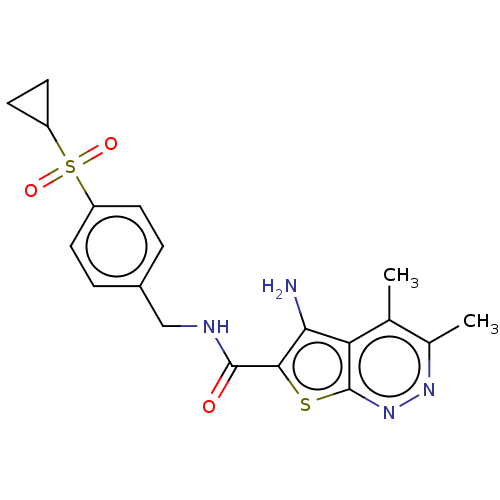
(CHEMBL3983700)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C3CC3)c(N)c2c1C Show InChI InChI=1S/C19H20N4O3S2/c1-10-11(2)22-23-19-15(10)16(20)17(27-19)18(24)21-9-12-3-5-13(6-4-12)28(25,26)14-7-8-14/h3-6,14H,7-9,20H2,1-2H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50207523
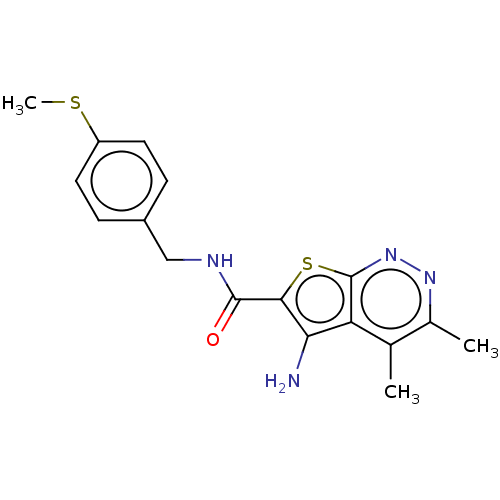
(CHEMBL3982695)Show InChI InChI=1S/C17H18N4OS2/c1-9-10(2)20-21-17-13(9)14(18)15(24-17)16(22)19-8-11-4-6-12(23-3)7-5-11/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50207524
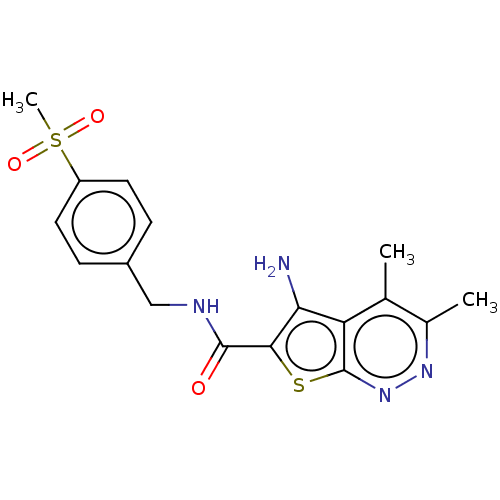
(CHEMBL3974708)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(C)(=O)=O)c(N)c2c1C Show InChI InChI=1S/C17H18N4O3S2/c1-9-10(2)20-21-17-13(9)14(18)15(25-17)16(22)19-8-11-4-6-12(7-5-11)26(3,23)24/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 363 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207524

(CHEMBL3974708)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(C)(=O)=O)c(N)c2c1C Show InChI InChI=1S/C17H18N4O3S2/c1-9-10(2)20-21-17-13(9)14(18)15(25-17)16(22)19-8-11-4-6-12(7-5-11)26(3,23)24/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207523

(CHEMBL3982695)Show InChI InChI=1S/C17H18N4OS2/c1-9-10(2)20-21-17-13(9)14(18)15(24-17)16(22)19-8-11-4-6-12(23-3)7-5-11/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylscopolamine from human M2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylscopolamine from human M3 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human M5 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M1 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M1 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M2 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to rat M3 receptor by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207524

(CHEMBL3974708)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(C)(=O)=O)c(N)c2c1C Show InChI InChI=1S/C17H18N4O3S2/c1-9-10(2)20-21-17-13(9)14(18)15(25-17)16(22)19-8-11-4-6-12(7-5-11)26(3,23)24/h4-7H,8,18H2,1-3H3,(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50207515

(CHEMBL3966208)Show SMILES CC(C)S(=O)(=O)c1ccc(CNC(=O)c2sc3nnc(C)c(C)c3c2N)cc1 Show InChI InChI=1S/C19H22N4O3S2/c1-10(2)28(25,26)14-7-5-13(6-8-14)9-21-18(24)17-16(20)15-11(3)12(4)22-23-19(15)27-17/h5-8,10H,9,20H2,1-4H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of rat M4 receptor expressed in CHO cells co-expressing Gqui5 by calcium mobilization assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data