

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50423774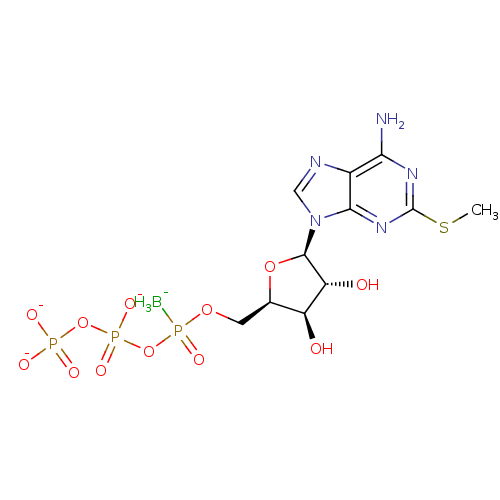 (CHEMBL2309024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor expressed in human HEK293 cells | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50308334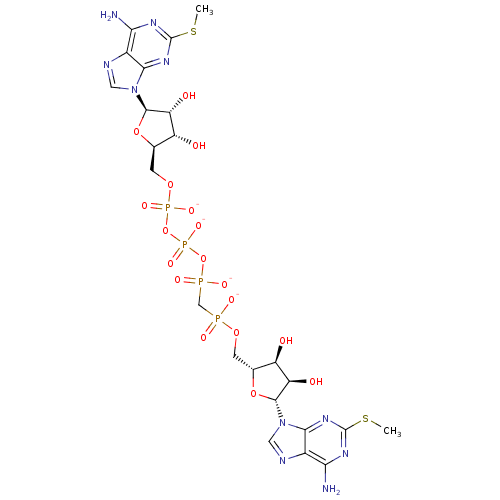 (CHEMBL591433 | Di-(2-MeS)-adenosine 5',5''-P1,P4,a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242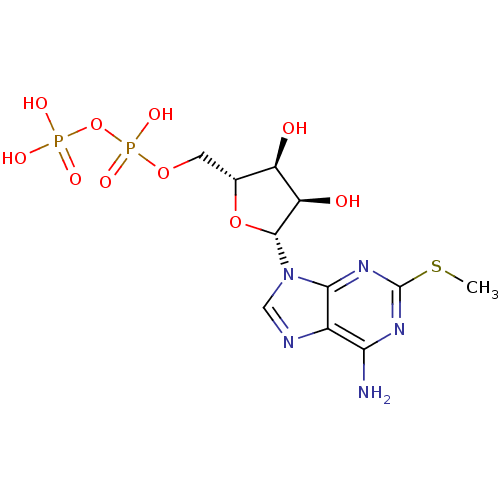 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50268012 ({[({[(2R,3S,4R,5R)-5-[6-amino-2-(methylsulfanyl)-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50308336 (CHEMBL591905 | {[5-(6-amino-9H-purin-9-yl)-3,4-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM2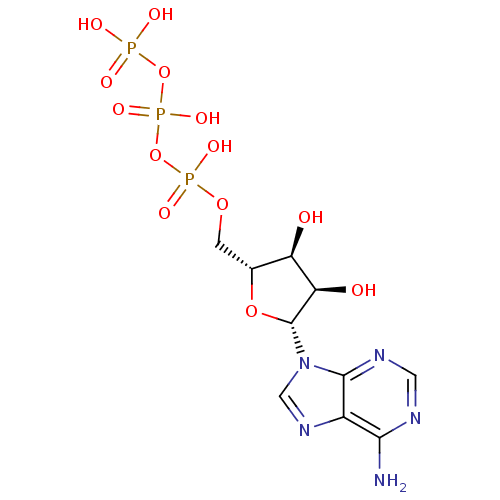 (({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor expressed in human HEK293 cells | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM31995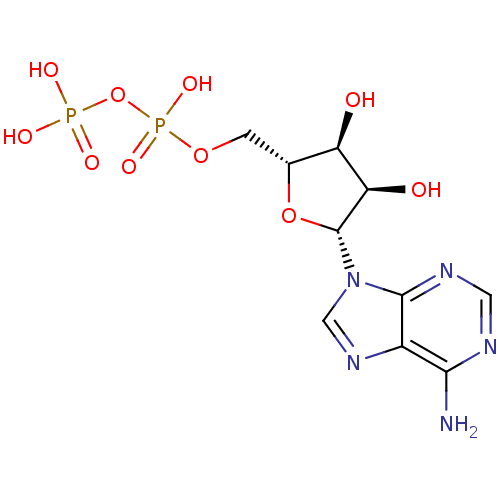 (ADP | Adenosine Diphosphate (ADP) | CHEMBL14830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50308335 (CHEMBL591434 | Di-(2-MeS)-adenosine 5',5''-P1,P4,b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||