

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313813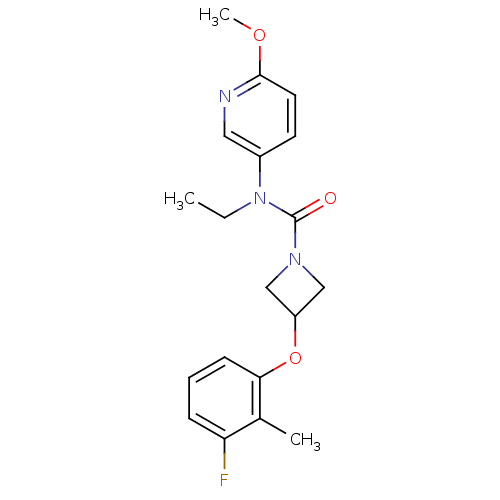 (CHEMBL1077300 | N-ethyl-3-(3-fluoro-2-methylphenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50305524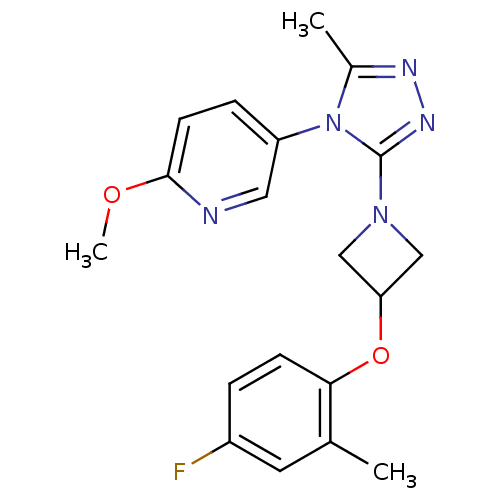 (5-(3-(3-(4-fluoro-2-methylphenoxy)azetidin-1-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313817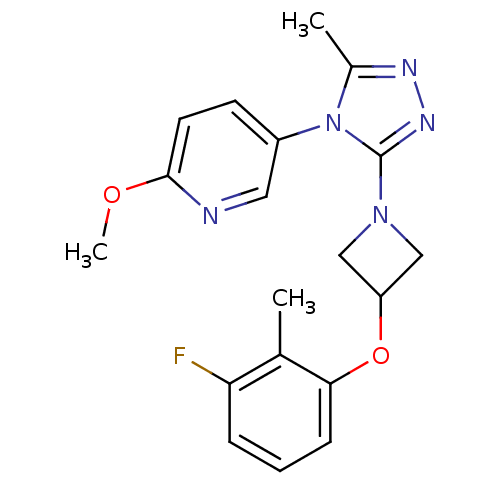 (5-(3-(3-(3-fluoro-2-methylphenoxy)azetidin-1-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50305523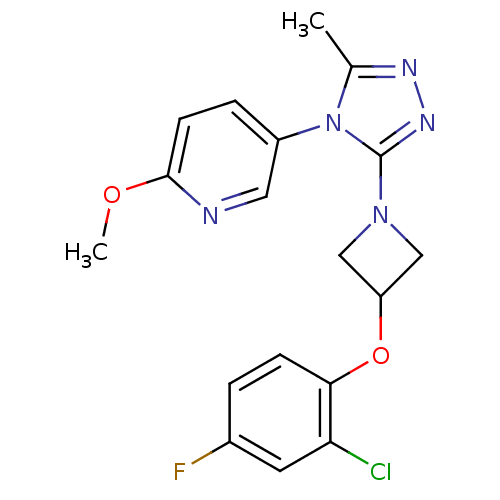 (5-(3-(3-(2-chloro-4-fluorophenoxy)azetidin-1-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313812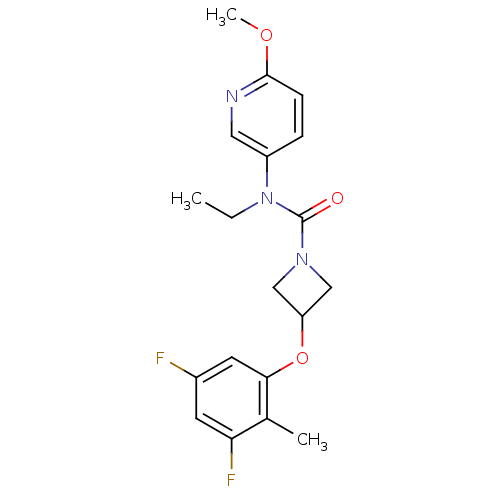 (3-(3,5-difluoro-2-methylphenoxy)-N-ethyl-N-(6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313816 (5-(3-(3-(3,5-difluoro-2-methylphenoxy)azetidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313815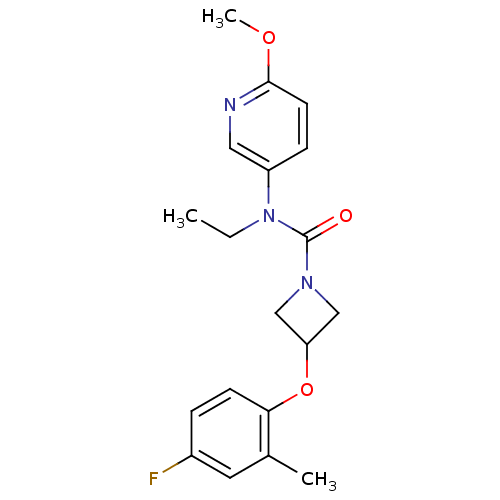 (CHEMBL1094050 | N-ethyl-3-(4-fluoro-2-methylphenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313814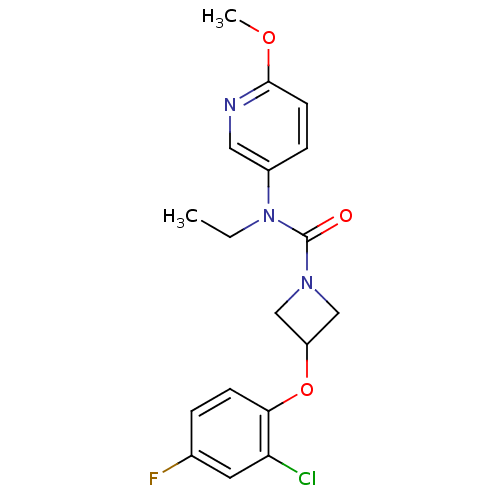 (3-(2-chloro-4-fluorophenoxy)-N-ethyl-N-(6-methoxyp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313811 (3-(2-chloro-4-fluorophenoxy)-N-(6-methoxypyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313810 (3-(4-fluoro-2-methylphenoxy)-N-(6-methoxypyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313808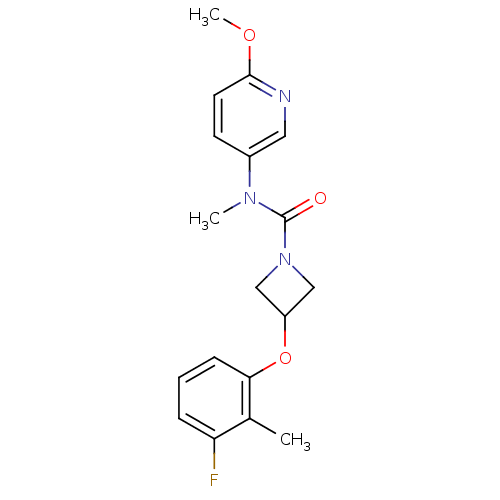 (3-(3-fluoro-2-methylphenoxy)-N-(6-methoxypyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50313809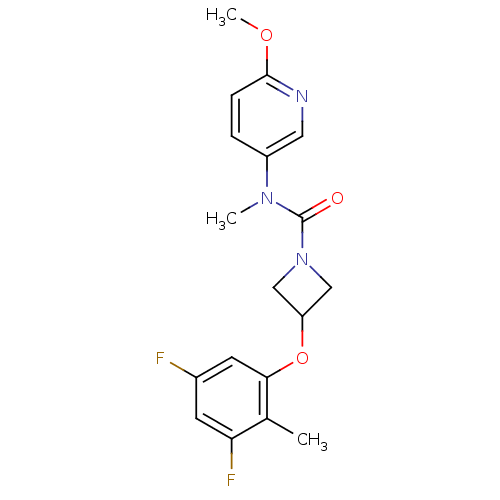 (3-(3,5-difluoro-2-methylphenoxy)-N-(6-methoxypyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human oxytocin receptor expressed in CHO cells by beta lactamase assay | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50305524 (5-(3-(3-(4-fluoro-2-methylphenoxy)azetidin-1-yl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 608 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313811 (3-(2-chloro-4-fluorophenoxy)-N-(6-methoxypyridin-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 779 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313810 (3-(4-fluoro-2-methylphenoxy)-N-(6-methoxypyridin-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50305523 (5-(3-(3-(2-chloro-4-fluorophenoxy)azetidin-1-yl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313817 (5-(3-(3-(3-fluoro-2-methylphenoxy)azetidin-1-yl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313816 (5-(3-(3-(3,5-difluoro-2-methylphenoxy)azetidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313813 (CHEMBL1077300 | N-ethyl-3-(3-fluoro-2-methylphenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313809 (3-(3,5-difluoro-2-methylphenoxy)-N-(6-methoxypyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50313808 (3-(3-fluoro-2-methylphenoxy)-N-(6-methoxypyridin-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1A receptor | Bioorg Med Chem Lett 20: 1851-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.143 BindingDB Entry DOI: 10.7270/Q2K074F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||