 Found 19 hits Enz. Inhib. hit(s) with all data for entry = 50031647
Found 19 hits Enz. Inhib. hit(s) with all data for entry = 50031647 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arginase-1
(Homo sapiens (Human)) | BDBM50316603
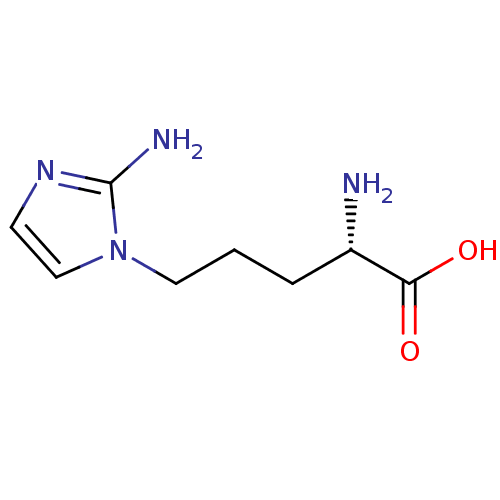
(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316607
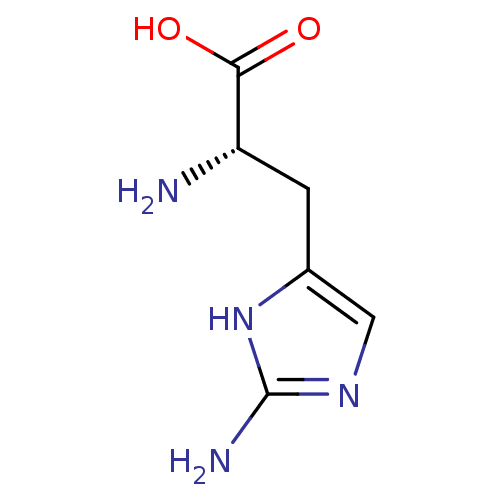
(2-amino-L-histidine | CHEMBL1099167 | L-2-aminohis...)Show InChI InChI=1S/C6H10N4O2/c7-4(5(11)12)1-3-2-9-6(8)10-3/h2,4H,1,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arginase-1
(Homo sapiens (Human)) | BDBM50316604
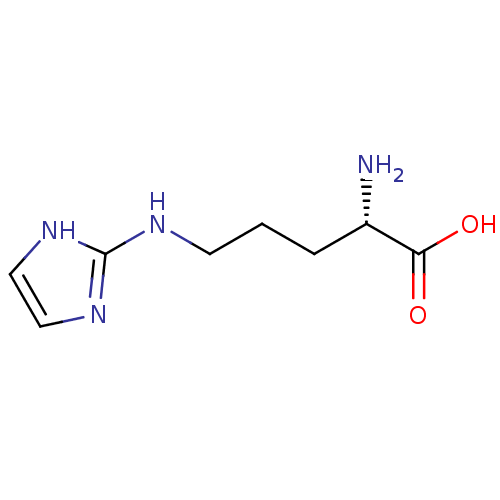
((S)-2-amino-5-(imidazol-2-ylamino)pentanoic acid |...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-3-10-8-11-4-5-12-8/h4-6H,1-3,9H2,(H,13,14)(H2,10,11,12)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316606

((2S)-2-amino-4-(2-amino-1H-imidazol-5-yl)butanoic ...)Show InChI InChI=1S/C7H12N4O2/c8-5(6(12)13)2-1-4-3-10-7(9)11-4/h3,5H,1-2,8H2,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arginase-1
(Homo sapiens (Human)) | BDBM50316608
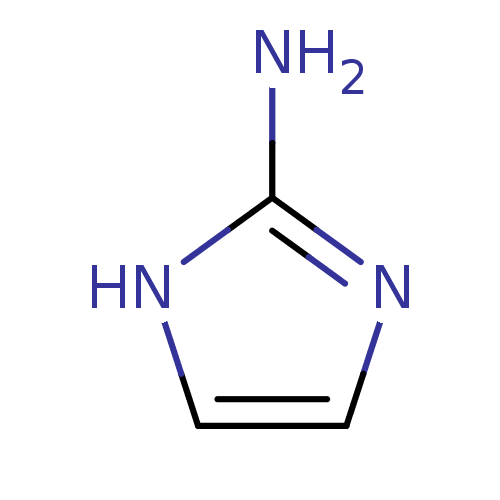
(1H-Imidazol-2-yl-ammonium | 1H-Imidazol-2-ylamine ...)Show InChI InChI=1S/C3H5N3/c4-3-5-1-2-6-3/h1-2H,(H3,4,5,6) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
Article
PubMed
| 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316605
(2-amino-5-(2-aminoimidazol-4-yl)pentanoic acid | C...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)3-1-2-5-4-11-8(10)12-5/h4,6H,1-3,9H2,(H,13,14)(H3,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50316607

(2-amino-L-histidine | CHEMBL1099167 | L-2-aminohis...)Show InChI InChI=1S/C6H10N4O2/c7-4(5(11)12)1-3-2-9-6(8)10-3/h2,4H,1,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition rat recombinant nNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50316604

((S)-2-amino-5-(imidazol-2-ylamino)pentanoic acid |...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-3-10-8-11-4-5-12-8/h4-6H,1-3,9H2,(H,13,14)(H2,10,11,12)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50316604

((S)-2-amino-5-(imidazol-2-ylamino)pentanoic acid |...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-3-10-8-11-4-5-12-8/h4-6H,1-3,9H2,(H,13,14)(H2,10,11,12)/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition rat recombinant nNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50316608

(1H-Imidazol-2-yl-ammonium | 1H-Imidazol-2-ylamine ...)Show InChI InChI=1S/C3H5N3/c4-3-5-1-2-6-3/h1-2H,(H3,4,5,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition rat recombinant nNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50316608

(1H-Imidazol-2-yl-ammonium | 1H-Imidazol-2-ylamine ...)Show InChI InChI=1S/C3H5N3/c4-3-5-1-2-6-3/h1-2H,(H3,4,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50316607

(2-amino-L-histidine | CHEMBL1099167 | L-2-aminohis...)Show InChI InChI=1S/C6H10N4O2/c7-4(5(11)12)1-3-2-9-6(8)10-3/h2,4H,1,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50316606

((2S)-2-amino-4-(2-amino-1H-imidazol-5-yl)butanoic ...)Show InChI InChI=1S/C7H12N4O2/c8-5(6(12)13)2-1-4-3-10-7(9)11-4/h3,5H,1-2,8H2,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition rat recombinant nNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50316606

((2S)-2-amino-4-(2-amino-1H-imidazol-5-yl)butanoic ...)Show InChI InChI=1S/C7H12N4O2/c8-5(6(12)13)2-1-4-3-10-7(9)11-4/h3,5H,1-2,8H2,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50316605

(2-amino-5-(2-aminoimidazol-4-yl)pentanoic acid | C...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)3-1-2-5-4-11-8(10)12-5/h4,6H,1-3,9H2,(H,13,14)(H3,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition rat recombinant nNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50316605

(2-amino-5-(2-aminoimidazol-4-yl)pentanoic acid | C...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)3-1-2-5-4-11-8(10)12-5/h4,6H,1-3,9H2,(H,13,14)(H3,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50316603

(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition rat recombinant nNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50316603

(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316603

(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by surface plasmon resonance assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data