

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50317180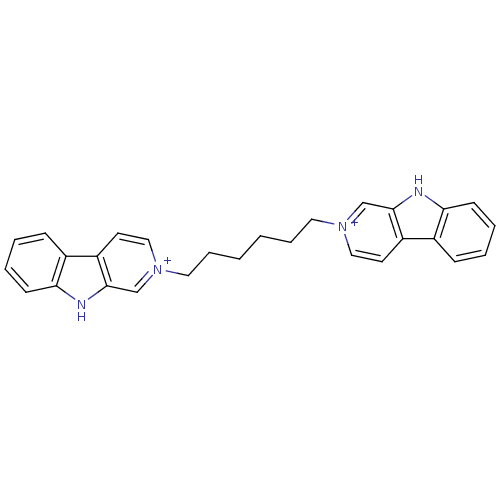 (2-[6-(beta-Carboline-2-ium-2-yl)hexyl]-beta-carbol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil from human NR1-1a/NR2B receptor expressed in mouse L13-E6 cells | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317179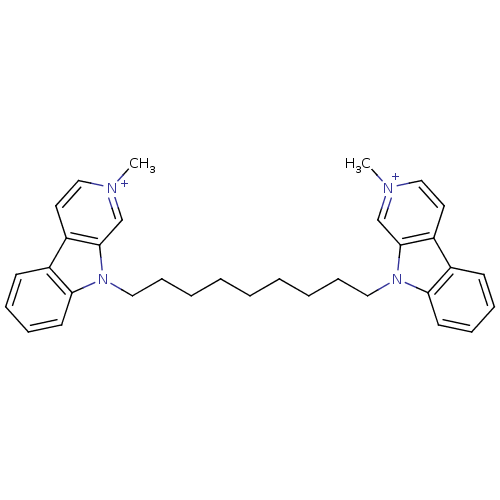 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.492 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.507 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317178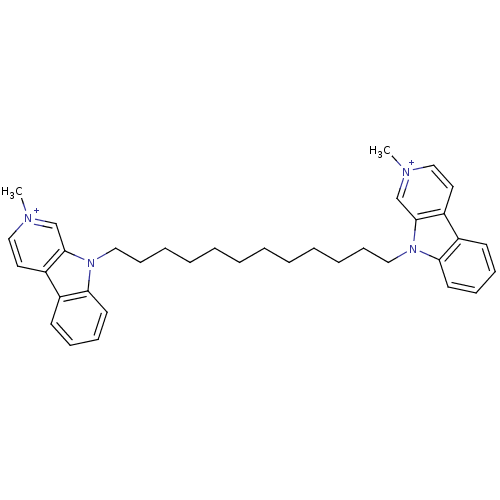 (2-Methyl-9-[12-(2-methyl-beta-carboline-9-yl)dodec...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317178 (2-Methyl-9-[12-(2-methyl-beta-carboline-9-yl)dodec...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317183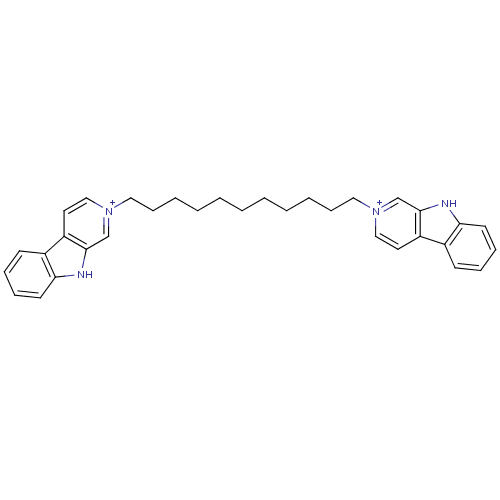 (2-[11-(beta-Carboline-2-ium-2-yl)undecyl]-beta-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317192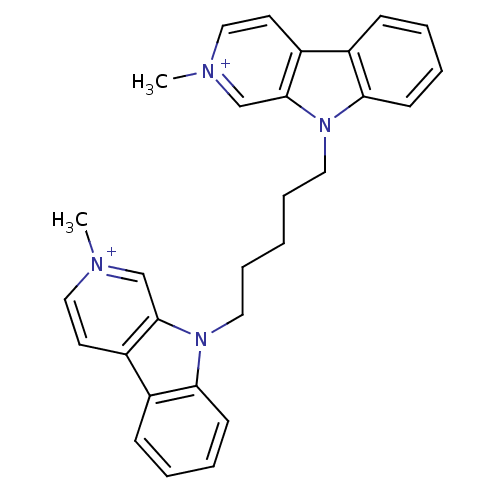 (2-Methyl-9-[5-(2-methyl-beta-carboline-9-yl)pentyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317182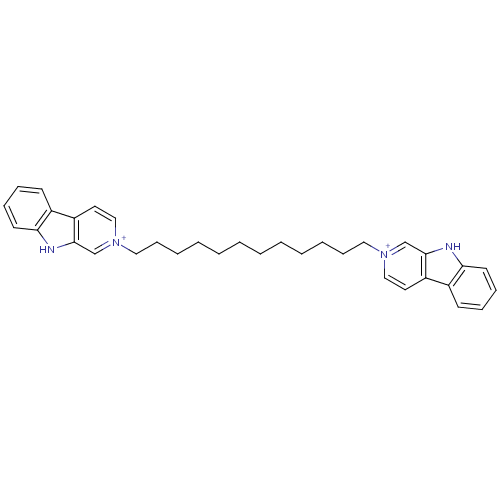 (2-[12-(beta-Carboline-2-ium-2-yl)dodecyl]-beta-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317193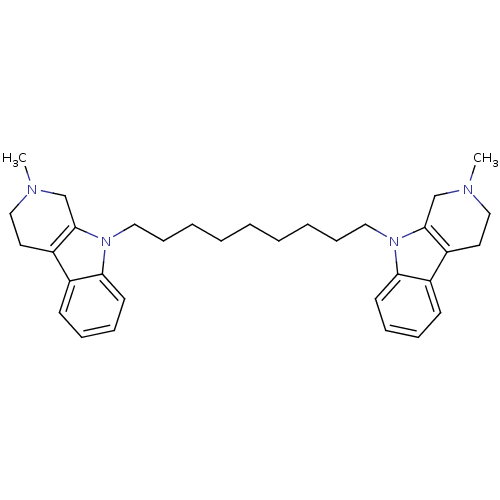 (2-Methyl-9-[9-(2-methyl-1,2,3,4-tetrahydro-beta-ca...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317193 (2-Methyl-9-[9-(2-methyl-1,2,3,4-tetrahydro-beta-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317188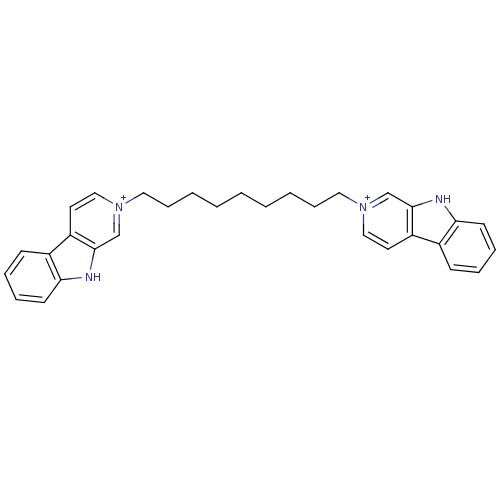 (2-[9-(beta-Carboline-2-ium-2-yl)nonyl]-beta-carbol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317184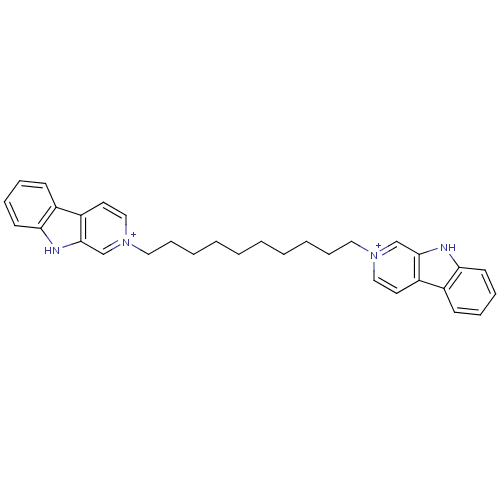 (2-[10-(beta-Carboline-2-ium-2-yl)decyl]-beta-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317186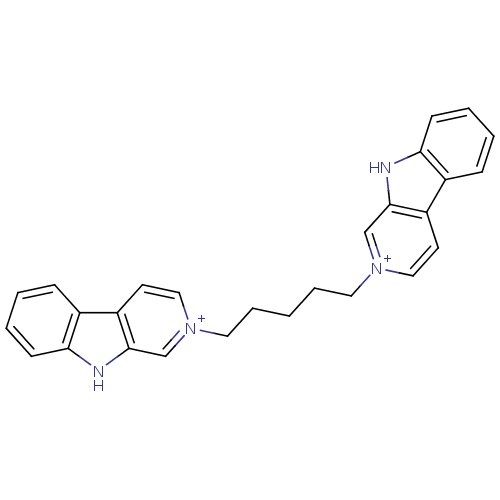 (2-[5-(beta-Carboline-2-ium-2-yl)pentyl]-beta-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317184 (2-[10-(beta-Carboline-2-ium-2-yl)decyl]-beta-carbo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317185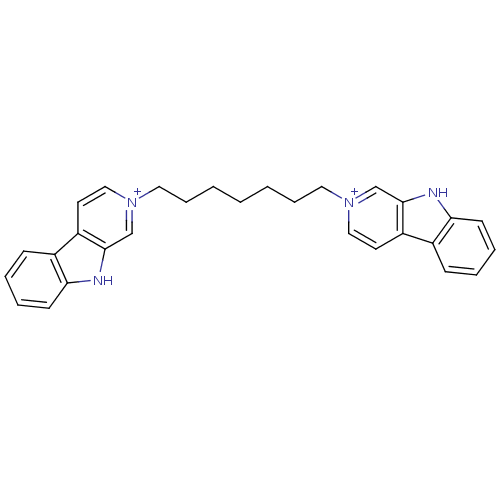 (2-[7-(beta-Carboline-2-ium-2-yl)heptyl]-beta-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317181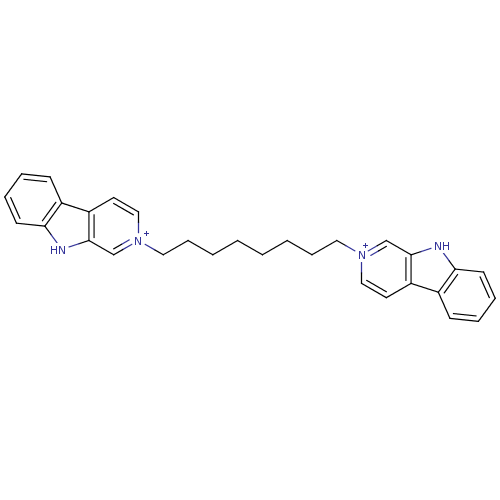 (2-[8-(beta-Carboline-2-ium-2-yl)octyl]-beta-carbol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317194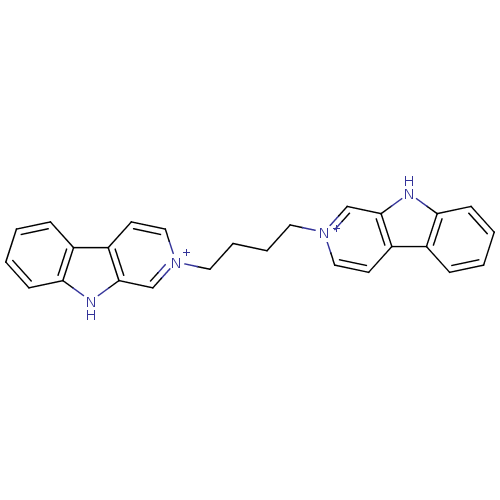 (2-[4-(beta-Carboline-2-ium-2-yl)butyl]-beta-carbol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317185 (2-[7-(beta-Carboline-2-ium-2-yl)heptyl]-beta-carbo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317182 (2-[12-(beta-Carboline-2-ium-2-yl)dodecyl]-beta-car...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317183 (2-[11-(beta-Carboline-2-ium-2-yl)undecyl]-beta-car...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317181 (2-[8-(beta-Carboline-2-ium-2-yl)octyl]-beta-carbol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317188 (2-[9-(beta-Carboline-2-ium-2-yl)nonyl]-beta-carbol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317195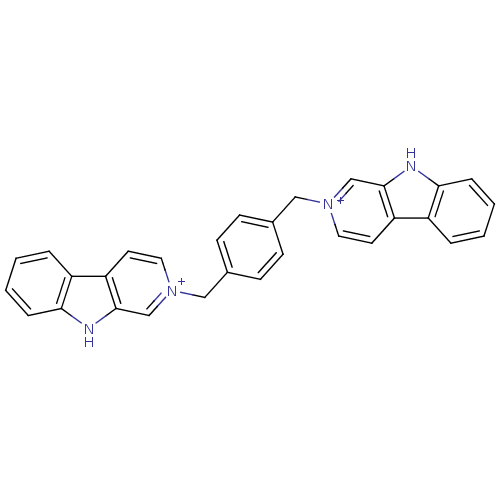 (2-[4-(beta-Carboline-2-ium-2-ylmethyl)benzyl]-beta...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317180 (2-[6-(beta-Carboline-2-ium-2-yl)hexyl]-beta-carbol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317191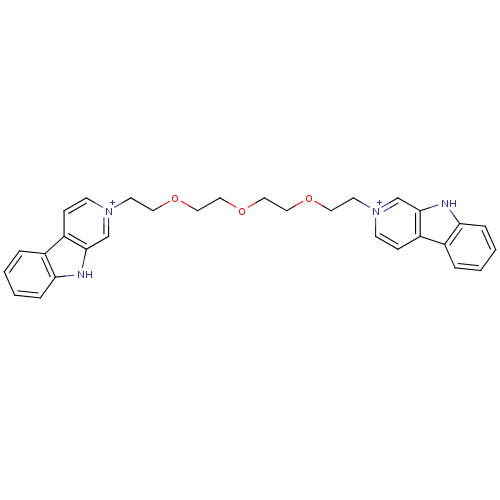 (2-[2-(2-{2-[2-(beta-Carboline-2-ium-2-yl)ethoxy]et...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317180 (2-[6-(beta-Carboline-2-ium-2-yl)hexyl]-beta-carbol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317194 (2-[4-(beta-Carboline-2-ium-2-yl)butyl]-beta-carbol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317192 (2-Methyl-9-[5-(2-methyl-beta-carboline-9-yl)pentyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317187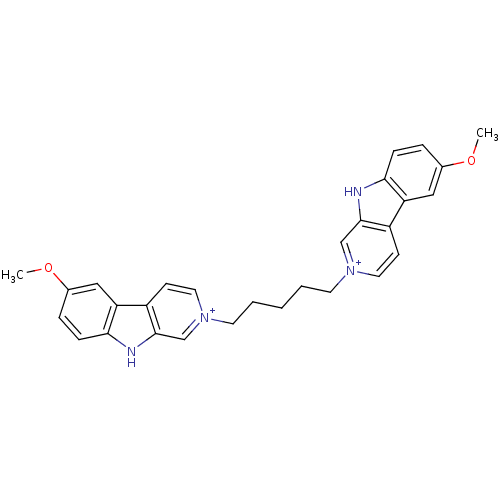 (6-Methoxy-2-[5-(6-methoxy-beta-carboline-2-ium-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317196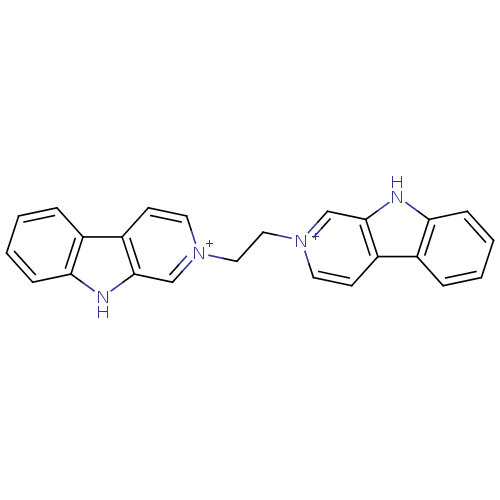 (2-[2-(beta-Carboline-2-ium-2-yl)ethyl]-beta-carbol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317196 (2-[2-(beta-Carboline-2-ium-2-yl)ethyl]-beta-carbol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317195 (2-[4-(beta-Carboline-2-ium-2-ylmethyl)benzyl]-beta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50269614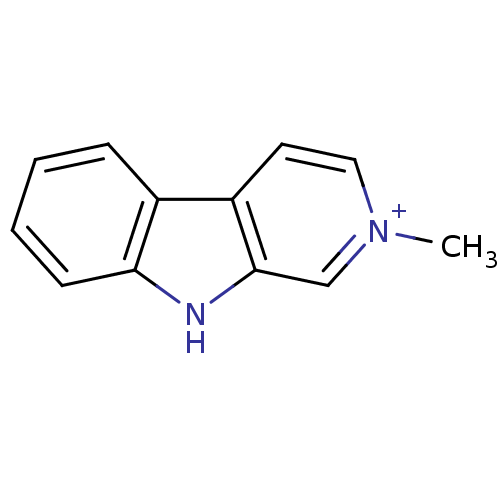 (2-Methyl-beta-carboline-2-ium iodide | 2-methyl-2H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317197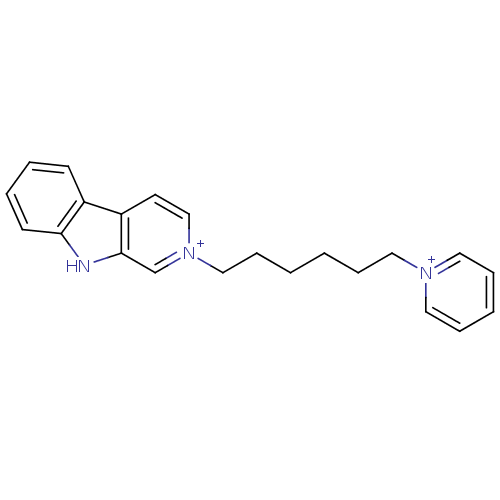 (2-(6-Pyridinium-1-yl-hexyl)-beta-carboline-2-ium d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 564 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317186 (2-[5-(beta-Carboline-2-ium-2-yl)pentyl]-beta-carbo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 595 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 636 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317189 (2-[3-(beta-Carboline-2-ium-2-yl)propyl]-beta-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 652 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317198 (2-Propyl-beta-carboline-2-ium iodide | CHEMBL10887...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 845 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317200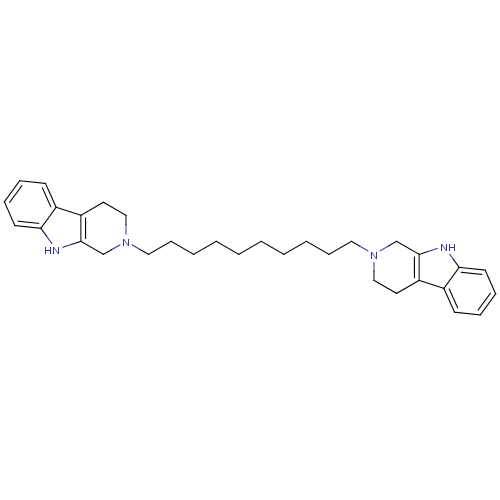 (2-[10-(1,2,3,4-Tetrahydro-beta-carboline-2-yl)decy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317204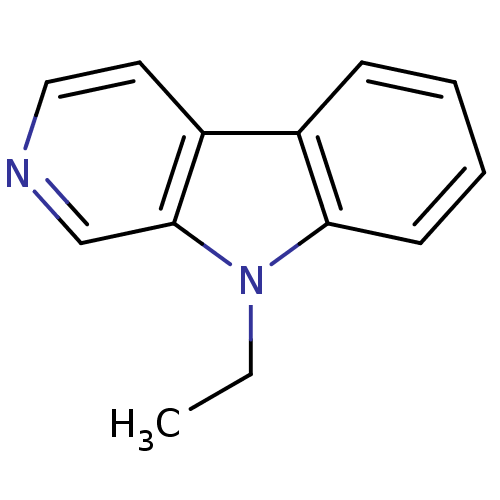 (9-Ethyl-beta-carboline | CHEMBL1097825) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317201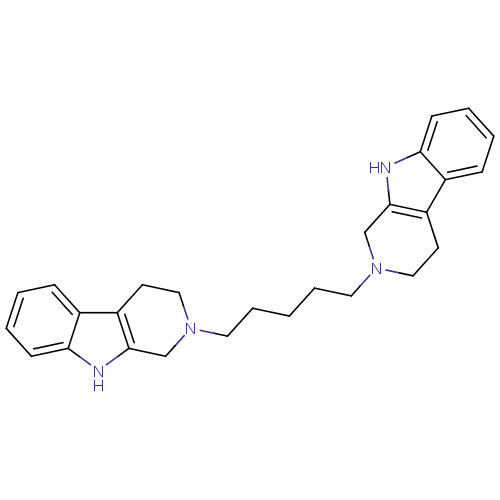 (2-[5-(1,2,3,4-Tetrahydro-beta-carboline-2-yl)penty...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317203 (9-[9-(beta-Carboline-9-yl)nonyl]-beta-carboline | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317202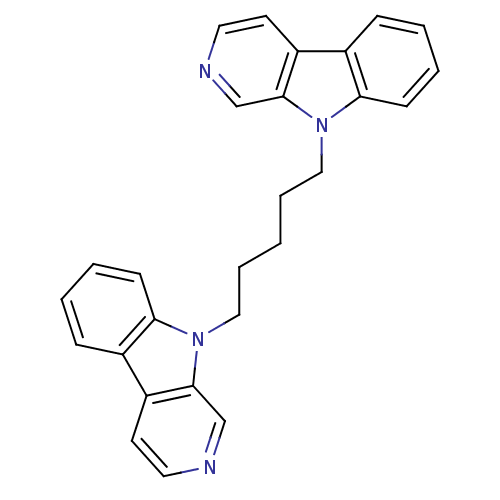 (9-[5-(beta-Carboline-9-yl)pentyl]-beta-carboline |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317201 (2-[5-(1,2,3,4-Tetrahydro-beta-carboline-2-yl)penty...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of dexamethasone-induced human NR1-1a/NR2A receptor-mediated excitotoxicity in (S)-glutamate/glycine-stimulated mouse L12-G10 cells assess... | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317190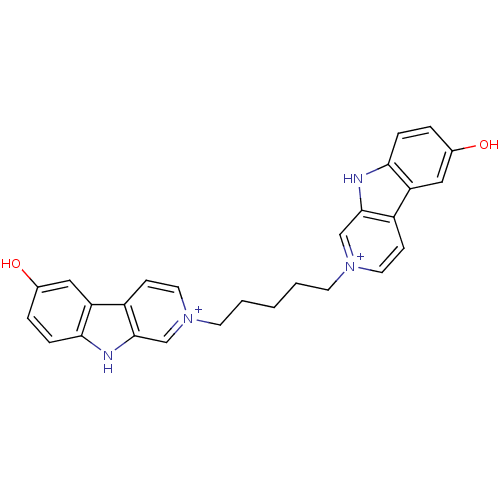 (6-Hydroxy-2-[5-(6-hydroxy-beta-carboline-2-ium-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50317182 (2-[12-(beta-Carboline-2-ium-2-yl)dodecyl]-beta-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of dexamethasone-induced human NR1-1a/NR2B receptor-mediated excitotoxicity in (S)-glutamate/glycine-stimulated mouse L13-E6 cells assesse... | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317199 (9-Ethyl-2-methyl-beta-carboline-2-ium iodide | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |