 Found 417 hits Enz. Inhib. hit(s) with all data for entry = 50031773
Found 417 hits Enz. Inhib. hit(s) with all data for entry = 50031773 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318731

(3-(1H-indol-3-yl)-N-(6-(1,2,3,4-tetrahydroacridin-...)Show SMILES O=C(CCc1c[nH]c2ccccc12)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H36N4O/c35-29(18-17-22-21-33-26-14-6-3-11-23(22)26)31-19-9-1-2-10-20-32-30-24-12-4-7-15-27(24)34-28-16-8-5-13-25(28)30/h3-4,6-7,11-12,14-15,21,33H,1-2,5,8-10,13,16-20H2,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318732
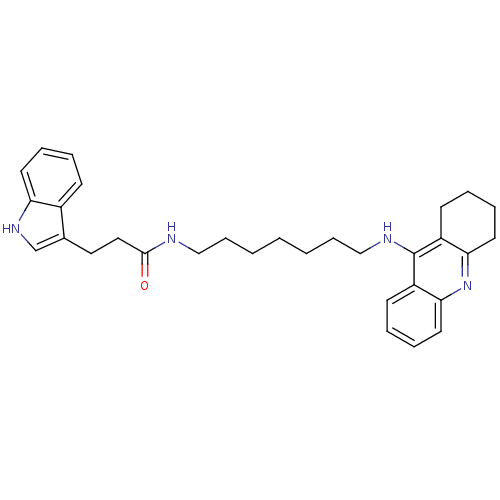
(3-(1H-indol-3-yl)-N-(7-(1,2,3,4-tetrahydroacridin-...)Show SMILES O=C(CCc1c[nH]c2ccccc12)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H38N4O/c36-30(19-18-23-22-34-27-15-7-4-12-24(23)27)32-20-10-2-1-3-11-21-33-31-25-13-5-8-16-28(25)35-29-17-9-6-14-26(29)31/h4-5,7-8,12-13,15-16,22,34H,1-3,6,9-11,14,17-21H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9054
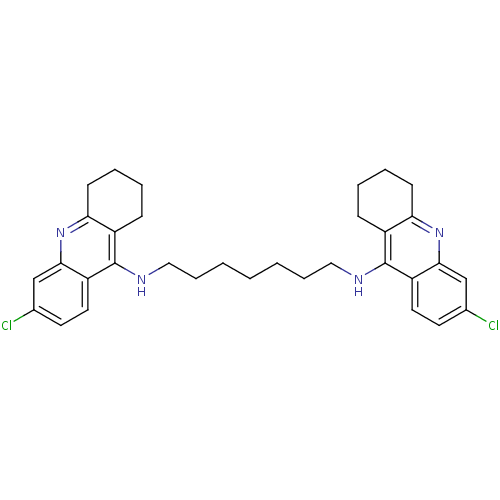
(6-chloro-N-{7-[(6-chloro-1,2,3,4-tetrahydroacridin...)Show SMILES Clc1ccc2c(NCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38Cl2N4/c34-22-14-16-26-30(20-22)38-28-12-6-4-10-24(28)32(26)36-18-8-2-1-3-9-19-37-33-25-11-5-7-13-29(25)39-31-21-23(35)15-17-27(31)33/h14-17,20-21H,1-13,18-19H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9035
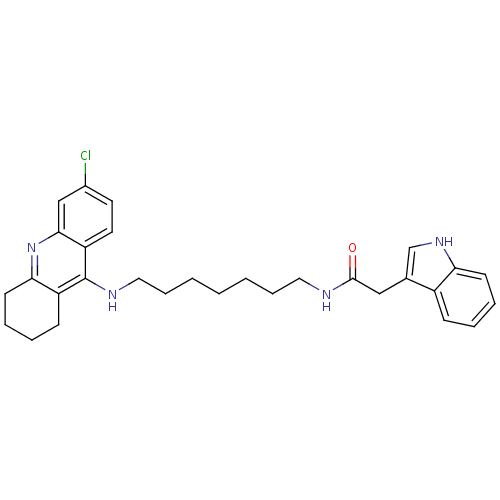
(Indole-Tacrine Heterodimer 18 | N-[7-(6-Chloro-1,2...)Show SMILES Clc1ccc2c(NCCCCCCCNC(=O)Cc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H35ClN4O/c31-22-14-15-25-28(19-22)35-27-13-7-5-11-24(27)30(25)33-17-9-3-1-2-8-16-32-29(36)18-21-20-34-26-12-6-4-10-23(21)26/h4,6,10,12,14-15,19-20,34H,1-3,5,7-9,11,13,16-18H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692
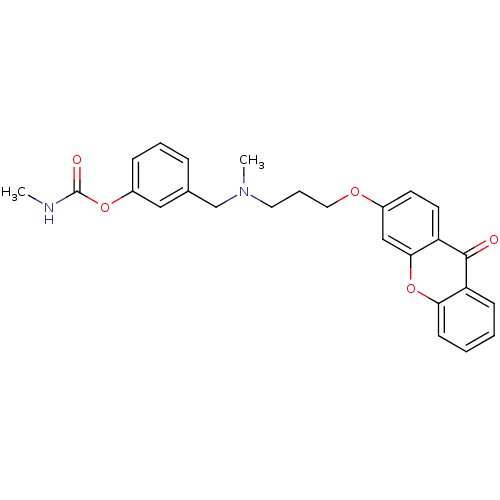
(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9037
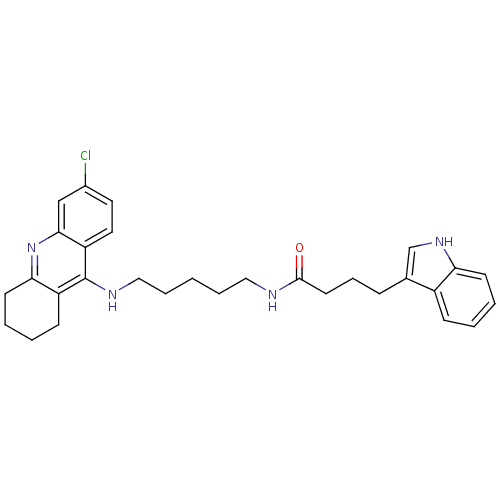
(Indole-Tacrine Heterodimer 20 | N-[5-(6-Chloro-1,2...)Show SMILES Clc1ccc2c(NCCCCCNC(=O)CCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H35ClN4O/c31-22-15-16-25-28(19-22)35-27-13-5-3-11-24(27)30(25)33-18-7-1-6-17-32-29(36)14-8-9-21-20-34-26-12-4-2-10-23(21)26/h2,4,10,12,15-16,19-20,34H,1,3,5-9,11,13-14,17-18H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9055
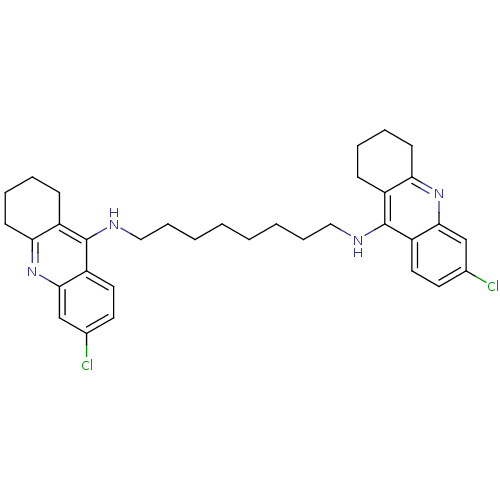
(6-chloro-N-{8-[(6-chloro-1,2,3,4-tetrahydroacridin...)Show SMILES Clc1ccc2c(NCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C34H40Cl2N4/c35-23-15-17-27-31(21-23)39-29-13-7-5-11-25(29)33(27)37-19-9-3-1-2-4-10-20-38-34-26-12-6-8-14-30(26)40-32-22-24(36)16-18-28(32)34/h15-18,21-22H,1-14,19-20H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10693
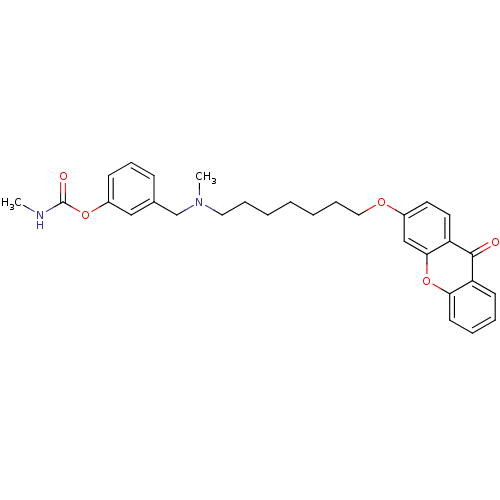
(3-{[methyl({7-[(9-oxo-9H-xanthen-3-yl)oxy]heptyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C30H34N2O5/c1-31-30(34)36-24-12-10-11-22(19-24)21-32(2)17-8-4-3-5-9-18-35-23-15-16-26-28(20-23)37-27-14-7-6-13-25(27)29(26)33/h6-7,10-16,19-20H,3-5,8-9,17-18,21H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
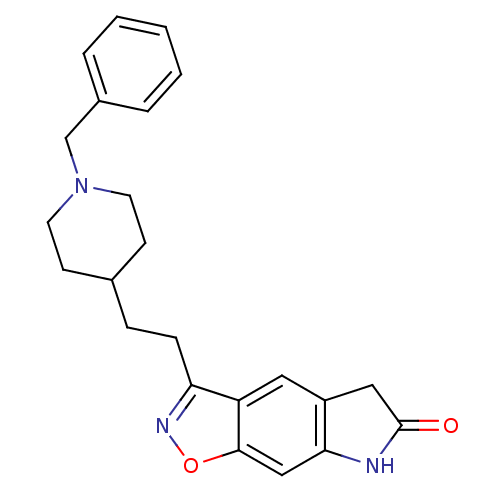
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318786

(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7,8-dihydroiso...)Show SMILES O=C1CNc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2C1 Show InChI InChI=1S/C24H27N3O2/c28-20-12-19-13-21-22(26-29-24(21)14-23(19)25-15-20)7-6-17-8-10-27(11-9-17)16-18-4-2-1-3-5-18/h1-5,13-14,17,25H,6-12,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9038
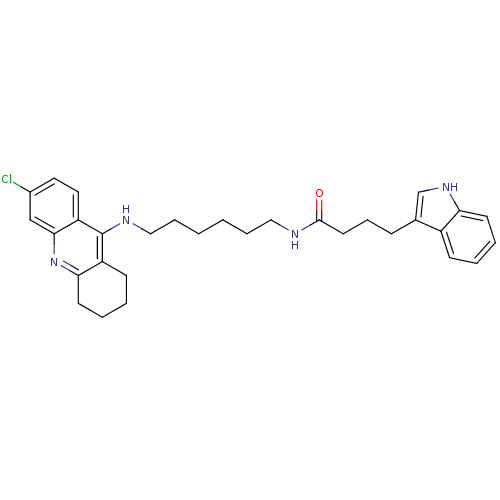
(Indole-Tacrine Heterodimer 21 | N-[6-(6-Chloro-1,2...)Show SMILES Clc1ccc2c(NCCCCCCNC(=O)CCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H37ClN4O/c32-23-16-17-26-29(20-23)36-28-14-6-4-12-25(28)31(26)34-19-8-2-1-7-18-33-30(37)15-9-10-22-21-35-27-13-5-3-11-24(22)27/h3,5,11,13,16-17,20-21,35H,1-2,4,6-10,12,14-15,18-19H2,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9024

(Indole-Tacrine Heterodimer 7 | N-[8-(6-Chloro-1,2,...)Show SMILES Clc1ccc2c(NCCCCCCCCNC(=O)CCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C32H39ClN4O/c33-24-16-17-27-30(21-24)37-29-14-8-6-12-26(29)32(27)35-20-10-4-2-1-3-9-19-34-31(38)18-15-23-22-36-28-13-7-5-11-25(23)28/h5,7,11,13,16-17,21-22,36H,1-4,6,8-10,12,14-15,18-20H2,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318727

(3-((methyl(7-(3-oxo-2-(3,4,5-trihydroxybenzylidene...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2ccc3C(=O)C(Oc3c2)=Cc2cc(O)c(O)c(O)c2)c1 |w:31.33| Show InChI InChI=1S/C32H36N2O8/c1-33-32(39)41-24-10-8-9-21(15-24)20-34(2)13-6-4-3-5-7-14-40-23-11-12-25-28(19-23)42-29(30(25)37)18-22-16-26(35)31(38)27(36)17-22/h8-12,15-19,35-36,38H,3-7,13-14,20H2,1-2H3,(H,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10932

(3-{[ethyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})a...)Show SMILES CCN(CCCOc1ccc2c(c1)oc1ccccc1c2=O)Cc1cccc(OC(=O)NC)c1 Show InChI InChI=1S/C27H28N2O5/c1-3-29(18-19-8-6-9-21(16-19)33-27(31)28-2)14-7-15-32-20-12-13-23-25(17-20)34-24-11-5-4-10-22(24)26(23)30/h4-6,8-13,16-17H,3,7,14-15,18H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
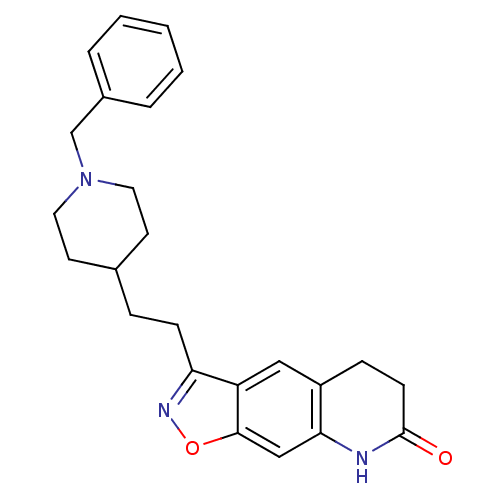
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9051

(6-fluoro-N-{7-[(6-fluoro-1,2,3,4-tetrahydroacridin...)Show SMILES Fc1ccc2c(NCCCCCCCNc3c4CCCCc4nc4cc(F)ccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38F2N4/c34-22-14-16-26-30(20-22)38-28-12-6-4-10-24(28)32(26)36-18-8-2-1-3-9-19-37-33-25-11-5-7-13-29(25)39-31-21-23(35)15-17-27(31)33/h14-17,20-21H,1-13,18-19H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9053

(6-chloro-N-{6-[(6-chloro-1,2,3,4-tetrahydroacridin...)Show SMILES Clc1ccc2c(NCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C32H36Cl2N4/c33-21-13-15-25-29(19-21)37-27-11-5-3-9-23(27)31(25)35-17-7-1-2-8-18-36-32-24-10-4-6-12-28(24)38-30-20-22(34)14-16-26(30)32/h13-16,19-20H,1-12,17-18H2,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9036
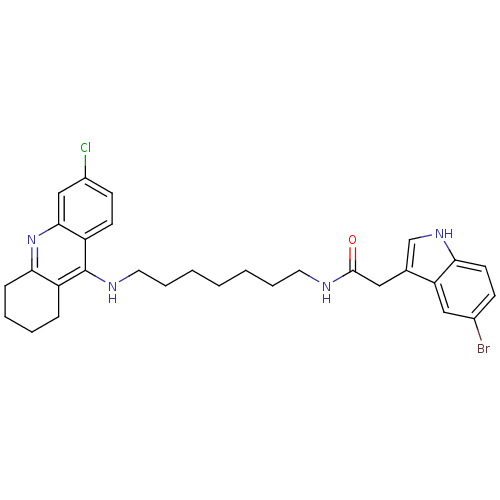
(2-(5-Bromo-1H-indol-3-yl)-N-[7-(6-chloro-1,2,3,4-t...)Show SMILES Clc1ccc2c(NCCCCCCCNC(=O)Cc3c[nH]c4ccc(Br)cc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H34BrClN4O/c31-21-10-13-26-25(17-21)20(19-35-26)16-29(37)33-14-6-2-1-3-7-15-34-30-23-8-4-5-9-27(23)36-28-18-22(32)11-12-24(28)30/h10-13,17-19,35H,1-9,14-16H2,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9029

(Indole-Tacrine Heterodimer 12 | N-[6-(6-Chloro-1,2...)Show SMILES Clc1ccc2c(NCCCCCCNC(=O)CCc3c[nH]c4ccc(cc34)C#N)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H34ClN5O/c32-23-11-12-25-29(18-23)37-28-8-4-3-7-24(28)31(25)35-16-6-2-1-5-15-34-30(38)14-10-22-20-36-27-13-9-21(19-33)17-26(22)27/h9,11-13,17-18,20,36H,1-8,10,14-16H2,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10938
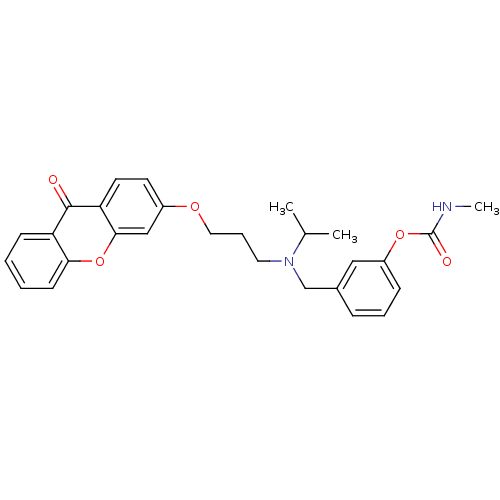
(3-[({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl}(propan-...)Show SMILES CNC(=O)Oc1cccc(CN(CCCOc2ccc3c(c2)oc2ccccc2c3=O)C(C)C)c1 Show InChI InChI=1S/C28H30N2O5/c1-19(2)30(18-20-8-6-9-22(16-20)34-28(32)29-3)14-7-15-33-21-12-13-24-26(17-21)35-25-11-5-4-10-23(25)27(24)31/h4-6,8-13,16-17,19H,7,14-15,18H2,1-3H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318734

(3-(2-(1H-indol-3-yl)ethoxy)-N-(6-(6-chloro-1,2,3,4...)Show SMILES Clc1ccc2c(NCCCCCCNC(=O)CCOCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C32H39ClN4O2/c33-24-13-14-27-30(21-24)37-29-12-6-4-10-26(29)32(27)35-18-8-2-1-7-17-34-31(38)16-20-39-19-15-23-22-36-28-11-5-3-9-25(23)28/h3,5,9,11,13-14,21-22,36H,1-2,4,6-8,10,12,15-20H2,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9052
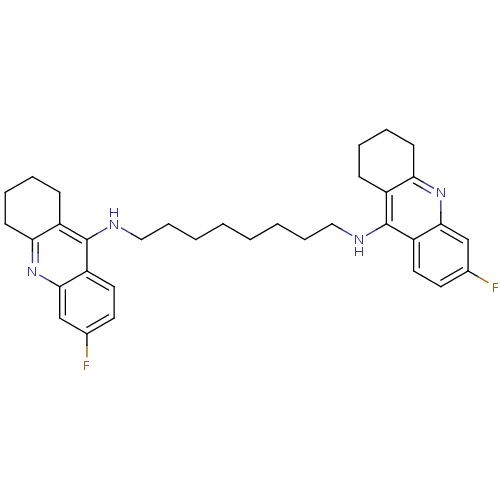
(6-fluoro-N-{8-[(6-fluoro-1,2,3,4-tetrahydroacridin...)Show SMILES Fc1ccc2c(NCCCCCCCCNc3c4CCCCc4nc4cc(F)ccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C34H40F2N4/c35-23-15-17-27-31(21-23)39-29-13-7-5-11-25(29)33(27)37-19-9-3-1-2-4-10-20-38-34-26-12-6-8-14-30(26)40-32-22-24(36)16-18-28(32)34/h15-18,21-22H,1-14,19-20H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039721
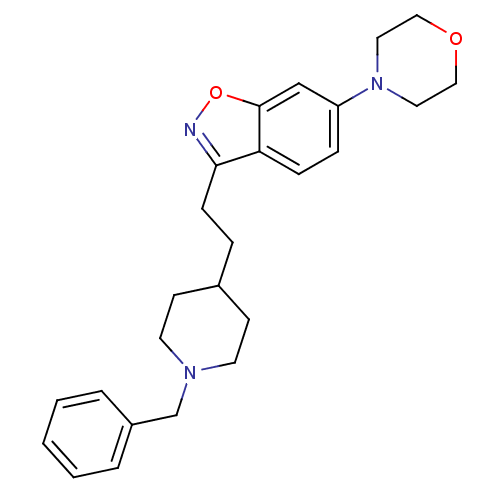
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...)Show SMILES C(Cc1noc2cc(ccc12)N1CCOCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O2/c1-2-4-21(5-3-1)19-27-12-10-20(11-13-27)6-9-24-23-8-7-22(18-25(23)30-26-24)28-14-16-29-17-15-28/h1-5,7-8,18,20H,6,9-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10913

(3-{[methyl({5-[(9-oxo-9H-xanthen-3-yl)oxy]pentyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C28H30N2O5/c1-29-28(32)34-22-10-8-9-20(17-22)19-30(2)15-6-3-7-16-33-21-13-14-24-26(18-21)35-25-12-5-4-11-23(25)27(24)31/h4-5,8-14,17-18H,3,6-7,15-16,19H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9050
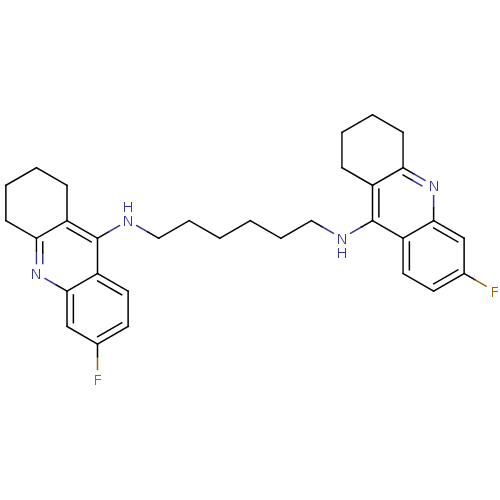
(6-fluoro-N-{6-[(6-fluoro-1,2,3,4-tetrahydroacridin...)Show SMILES Fc1ccc2c(NCCCCCCNc3c4CCCCc4nc4cc(F)ccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C32H36F2N4/c33-21-13-15-25-29(19-21)37-27-11-5-3-9-23(27)31(25)35-17-7-1-2-8-18-36-32-24-10-4-6-12-28(24)38-30-20-22(34)14-16-26(30)32/h13-16,19-20H,1-12,17-18H2,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
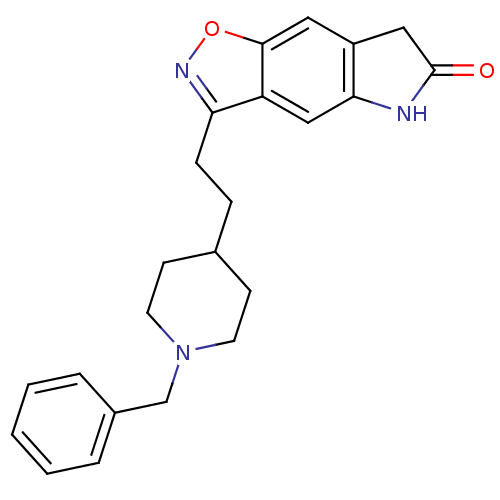
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10920
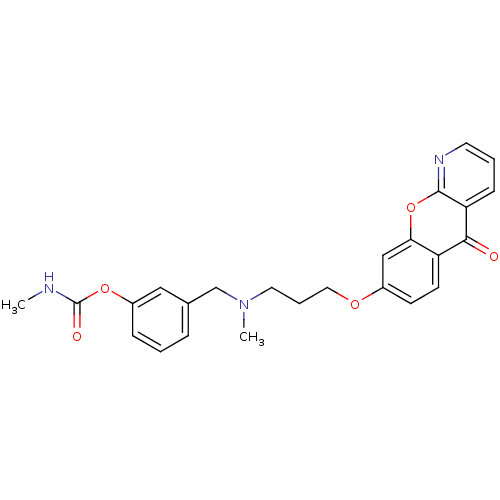
(3-({methyl[3-({5-oxo-5H-chromeno[2,3-b]pyridin-8-y...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ncccc2c3=O)c1 Show InChI InChI=1S/C25H25N3O5/c1-26-25(30)32-19-7-3-6-17(14-19)16-28(2)12-5-13-31-18-9-10-20-22(15-18)33-24-21(23(20)29)8-4-11-27-24/h3-4,6-11,14-15H,5,12-13,16H2,1-2H3,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004016
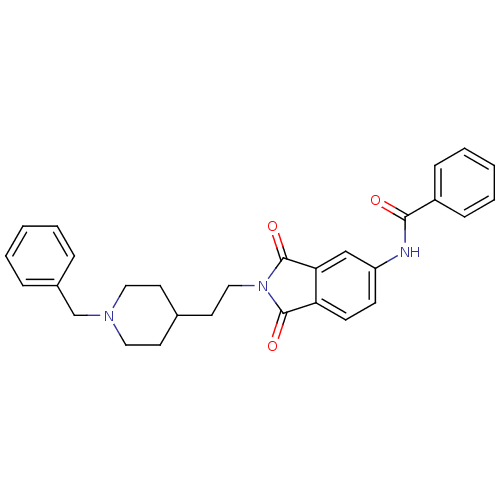
(CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...)Show SMILES O=C(Nc1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1)c1ccccc1 Show InChI InChI=1S/C29H29N3O3/c33-27(23-9-5-2-6-10-23)30-24-11-12-25-26(19-24)29(35)32(28(25)34)18-15-21-13-16-31(17-14-21)20-22-7-3-1-4-8-22/h1-12,19,21H,13-18,20H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9057
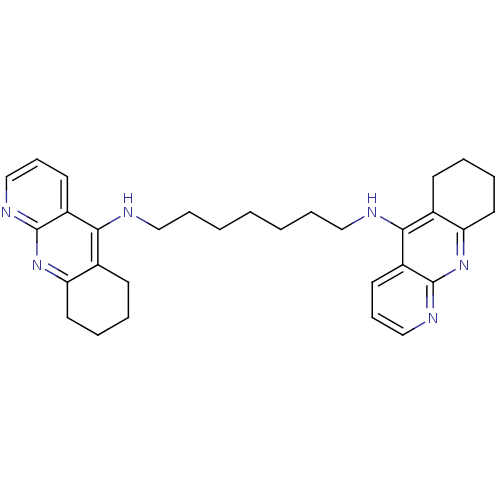
(Homodimeric Tacrine Analog 3k | N,N-Bis-(1,2,3,4-t...)Show SMILES C(CCCNc1c2CCCCc2nc2ncccc12)CCCNc1c2CCCCc2nc2ncccc12 Show InChI InChI=1S/C31H38N6/c1(2-8-18-32-28-22-12-4-6-16-26(22)36-30-24(28)14-10-20-34-30)3-9-19-33-29-23-13-5-7-17-27(23)37-31-25(29)15-11-21-35-31/h10-11,14-15,20-21H,1-9,12-13,16-19H2,(H,32,34,36)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10710
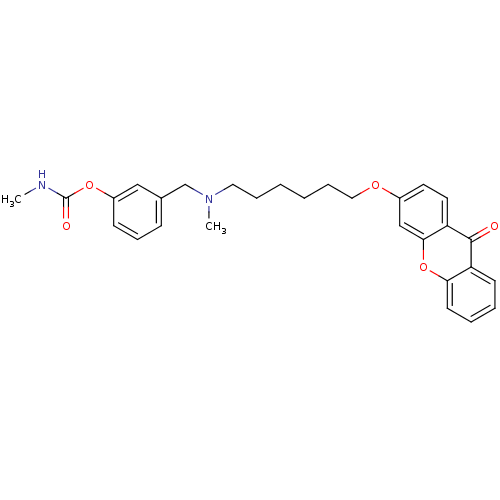
(3-{[methyl({6-[(9-oxo-9H-xanthen-3-yl)oxy]hexyl})a...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C29H32N2O5/c1-30-29(33)35-23-11-9-10-21(18-23)20-31(2)16-7-3-4-8-17-34-22-14-15-25-27(19-22)36-26-13-6-5-12-24(26)28(25)32/h5-6,9-15,18-19H,3-4,7-8,16-17,20H2,1-2H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
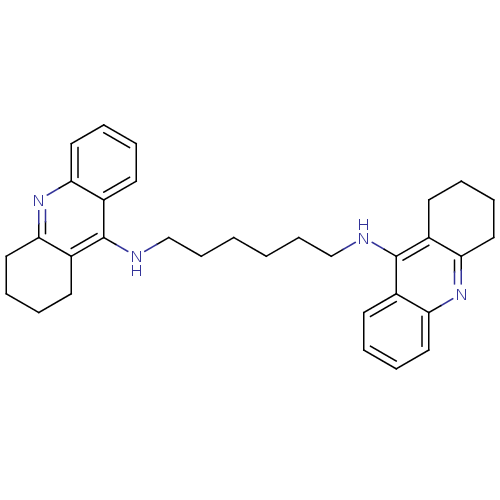
(Homo sapiens (Human)) | BDBM9047
(Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C32H38N4/c1(11-21-33-31-23-13-3-7-17-27(23)35-28-18-8-4-14-24(28)31)2-12-22-34-32-25-15-5-9-19-29(25)36-30-20-10-6-16-26(30)32/h3,5,7,9,13,15,17,19H,1-2,4,6,8,10-12,14,16,18,20-22H2,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
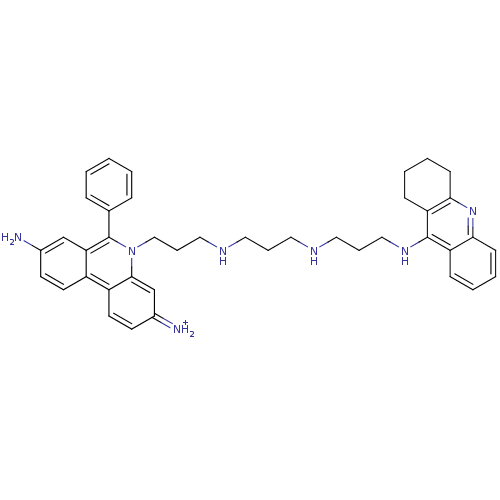
(Homo sapiens (Human)) | BDBM50158582
(3,8-Diamino-6-phenyl-5-(3-{3-[3-(1,2,3,4-tetrahydr...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCNCCCNCCCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C41H47N7/c42-30-17-19-32-33-20-18-31(43)28-39(33)48(41(36(32)27-30)29-11-2-1-3-12-29)26-10-24-45-22-8-21-44-23-9-25-46-40-34-13-4-6-15-37(34)47-38-16-7-5-14-35(38)40/h1-4,6,11-13,15,17-20,27-28,43-45H,5,7-10,14,16,21-26,42H2,(H,46,47)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
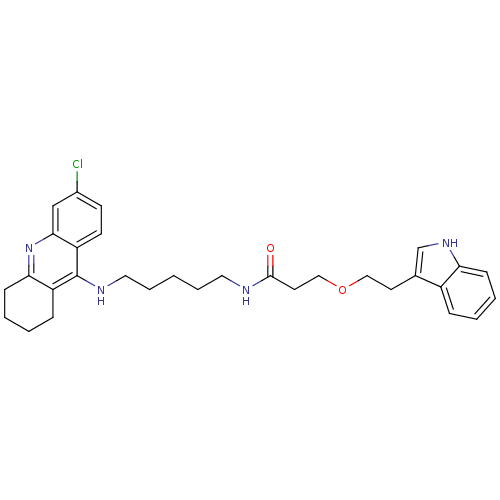
(Homo sapiens (Human)) | BDBM50318733
(3-(2-(1H-indol-3-yl)ethoxy)-N-(5-(6-chloro-1,2,3,4...)Show SMILES Clc1ccc2c(NCCCCCNC(=O)CCOCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H37ClN4O2/c32-23-12-13-26-29(20-23)36-28-11-5-3-9-25(28)31(26)34-17-7-1-6-16-33-30(37)15-19-38-18-14-22-21-35-27-10-4-2-8-24(22)27/h2,4,8,10,12-13,20-21,35H,1,3,5-7,9,11,14-19H2,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
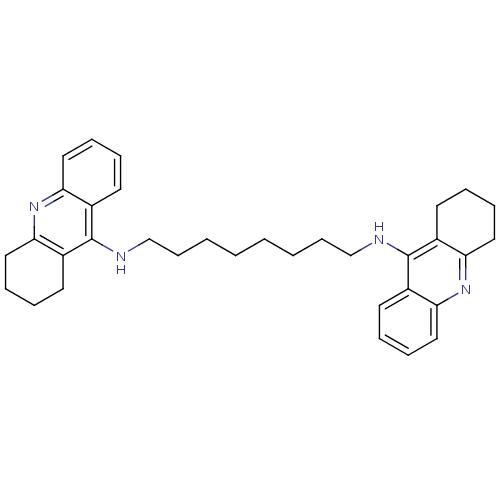
(Homo sapiens (Human)) | BDBM8964
(CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...)Show SMILES C(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H42N4/c1(3-13-23-35-33-25-15-5-9-19-29(25)37-30-20-10-6-16-26(30)33)2-4-14-24-36-34-27-17-7-11-21-31(27)38-32-22-12-8-18-28(32)34/h5,7,9,11,15,17,19,21H,1-4,6,8,10,12-14,16,18,20,22-24H2,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9061

(Homodimeric Tacrine Analog 4c | N,N-Bis-(2,3,4,5-t...)Show SMILES C(CCCCNc1c2CCCCCc2nc2ccccc12)CCCNc1c2CCCCCc2nc2ccccc12 Show InChI InChI=1S/C36H46N4/c1(3-15-25-37-35-27-17-7-5-9-21-31(27)39-33-23-13-11-19-29(33)35)2-4-16-26-38-36-28-18-8-6-10-22-32(28)40-34-24-14-12-20-30(34)36/h11-14,19-20,23-24H,1-10,15-18,21-22,25-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318728

(3-((methyl(7-(1-oxo-2-(3,4,5-trihydroxybenzylidene...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2ccc3C(=O)C(Cc3c2)=Cc2cc(O)c(O)c(O)c2)c1 |w:31.33| Show InChI InChI=1S/C33H38N2O7/c1-34-33(40)42-27-10-8-9-22(16-27)21-35(2)13-6-4-3-5-7-14-41-26-11-12-28-24(20-26)19-25(31(28)38)15-23-17-29(36)32(39)30(37)18-23/h8-12,15-18,20,36-37,39H,3-7,13-14,19,21H2,1-2H3,(H,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9058
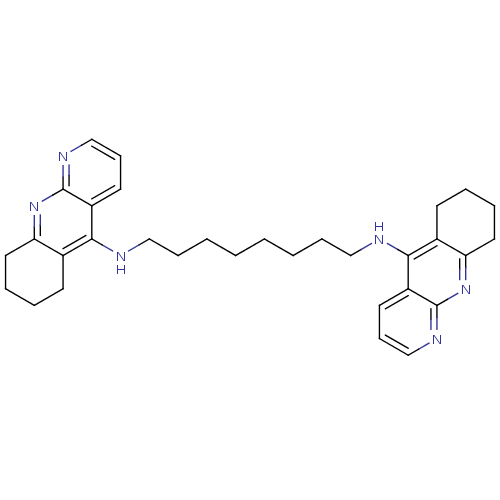
(Homodimeric Tacrine Analog 3m | N,N-Bis-(1,2,3,4-t...)Show SMILES C(CCCCNc1c2CCCCc2nc2ncccc12)CCCNc1c2CCCCc2nc2ncccc12 Show InChI InChI=1S/C32H40N6/c1(3-9-19-33-29-23-13-5-7-17-27(23)37-31-25(29)15-11-21-35-31)2-4-10-20-34-30-24-14-6-8-18-28(24)38-32-26(30)16-12-22-36-32/h11-12,15-16,21-22H,1-10,13-14,17-20H2,(H,33,35,37)(H,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10701

(3-{[(7-{[(2Z)-2-benzylidene-3-oxo-2,3-dihydro-1-be...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2ccc3C(=O)C(Oc3c2)=Cc2ccccc2)c1 |w:31.33| Show InChI InChI=1S/C32H36N2O5/c1-33-32(36)38-27-15-11-14-25(20-27)23-34(2)18-9-4-3-5-10-19-37-26-16-17-28-29(22-26)39-30(31(28)35)21-24-12-7-6-8-13-24/h6-8,11-17,20-22H,3-5,9-10,18-19,23H2,1-2H3,(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10699
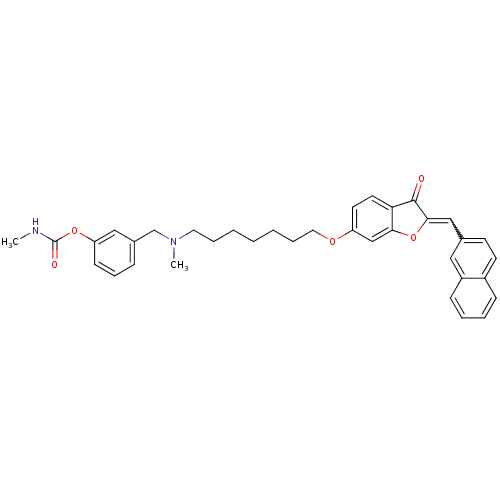
(3-{[methyl(7-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2ccc3C(=O)C(Oc3c2)=Cc2ccc3ccccc3c2)c1 |w:31.33| Show InChI InChI=1S/C36H38N2O5/c1-37-36(40)42-31-14-10-11-27(22-31)25-38(2)19-8-4-3-5-9-20-41-30-17-18-32-33(24-30)43-34(35(32)39)23-26-15-16-28-12-6-7-13-29(28)21-26/h6-7,10-18,21-24H,3-5,8-9,19-20,25H2,1-2H3,(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10698
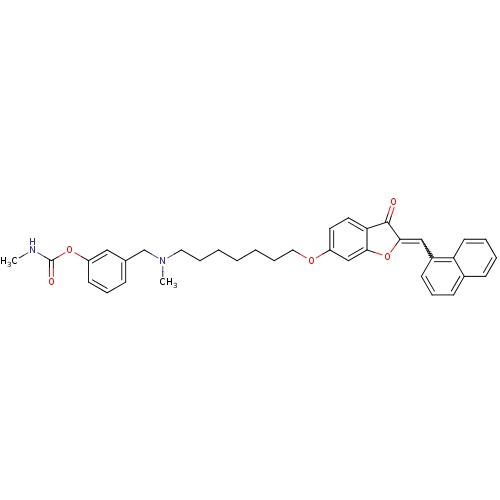
(3-{[methyl(7-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCCCCCOc2ccc3C(=O)C(Oc3c2)=Cc2cccc3ccccc23)c1 |w:31.33| Show InChI InChI=1S/C36H38N2O5/c1-37-36(40)42-30-16-10-12-26(22-30)25-38(2)20-8-4-3-5-9-21-41-29-18-19-32-33(24-29)43-34(35(32)39)23-28-15-11-14-27-13-6-7-17-31(27)28/h6-7,10-19,22-24H,3-5,8-9,20-21,25H2,1-2H3,(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318785
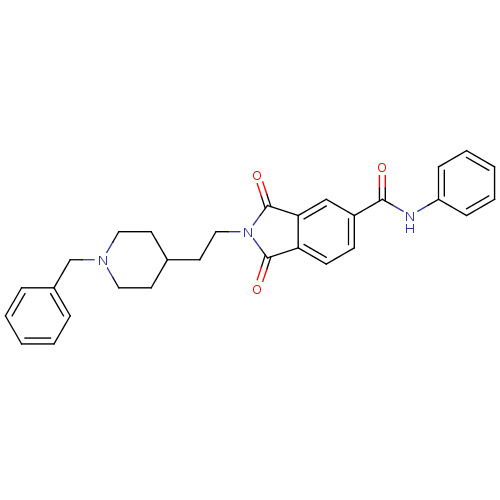
(2-(2-(1-benzylpiperidin-4-yl)ethyl)-1,3-dioxo-N-ph...)Show SMILES O=C(Nc1ccccc1)c1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 Show InChI InChI=1S/C29H29N3O3/c33-27(30-24-9-5-2-6-10-24)23-11-12-25-26(19-23)29(35)32(28(25)34)18-15-21-13-16-31(17-14-21)20-22-7-3-1-4-8-22/h1-12,19,21H,13-18,20H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004001
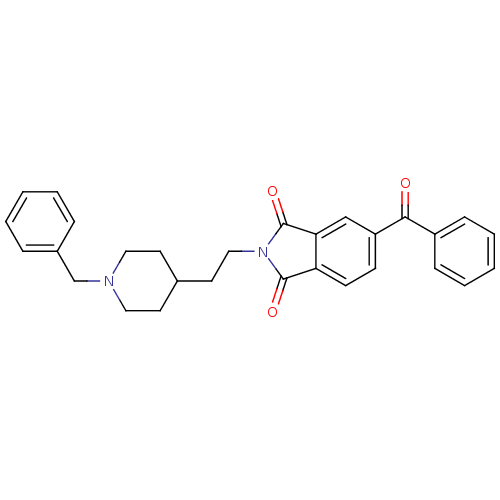
(5-Benzoyl-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-is...)Show SMILES O=C(c1ccccc1)c1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 Show InChI InChI=1S/C29H28N2O3/c32-27(23-9-5-2-6-10-23)24-11-12-25-26(19-24)29(34)31(28(25)33)18-15-21-13-16-30(17-14-21)20-22-7-3-1-4-8-22/h1-12,19,21H,13-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318720
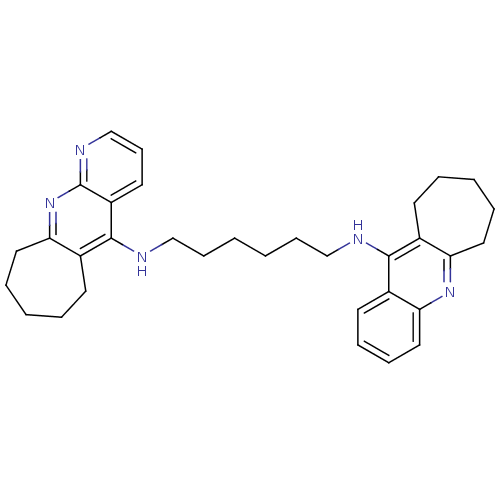
(CHEMBL1083488 | N1-(7,8,9,10-tetrahydro-6H-cyclohe...)Show SMILES C(CCCNc1c2CCCCCc2nc2ncccc12)CCNc1c2CCCCCc2nc2ccccc12 Show InChI InChI=1S/C33H41N5/c1(11-21-34-31-24-14-5-3-7-18-28(24)37-29-20-10-9-16-25(29)31)2-12-22-35-32-26-15-6-4-8-19-30(26)38-33-27(32)17-13-23-36-33/h9-10,13,16-17,20,23H,1-8,11-12,14-15,18-19,21-22H2,(H,34,37)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9060
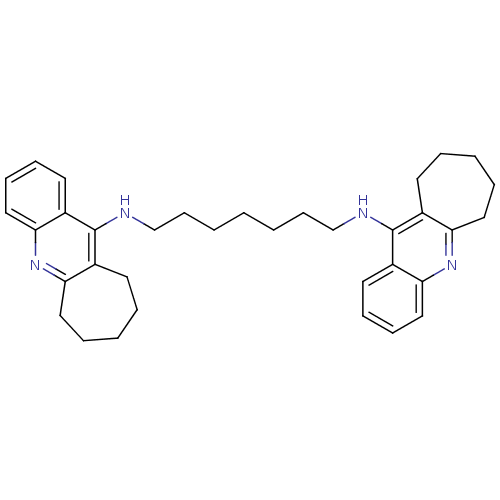
(Homodimeric Tacrine Analog 4b | N,N-Bis-(2,3,4,5-t...)Show SMILES C(CCCNc1c2CCCCCc2nc2ccccc12)CCCNc1c2CCCCCc2nc2ccccc12 Show InChI InChI=1S/C35H44N4/c1(2-14-24-36-34-26-16-6-4-8-20-30(26)38-32-22-12-10-18-28(32)34)3-15-25-37-35-27-17-7-5-9-21-31(27)39-33-23-13-11-19-29(33)35/h10-13,18-19,22-23H,1-9,14-17,20-21,24-25H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004035
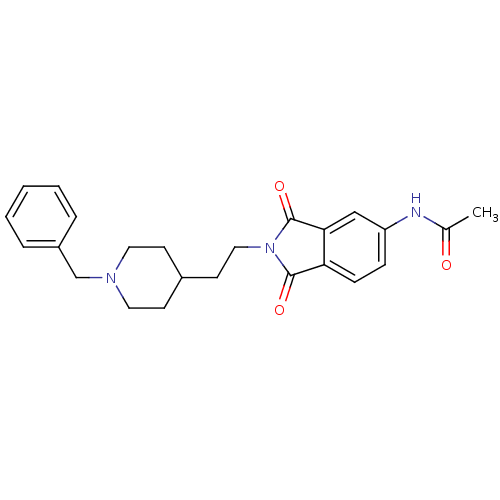
(CHEMBL433678 | N-(2-(2-(1-benzylpiperidin-4-yl)eth...)Show SMILES CC(=O)Nc1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 Show InChI InChI=1S/C24H27N3O3/c1-17(28)25-20-7-8-21-22(15-20)24(30)27(23(21)29)14-11-18-9-12-26(13-10-18)16-19-5-3-2-4-6-19/h2-8,15,18H,9-14,16H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10919
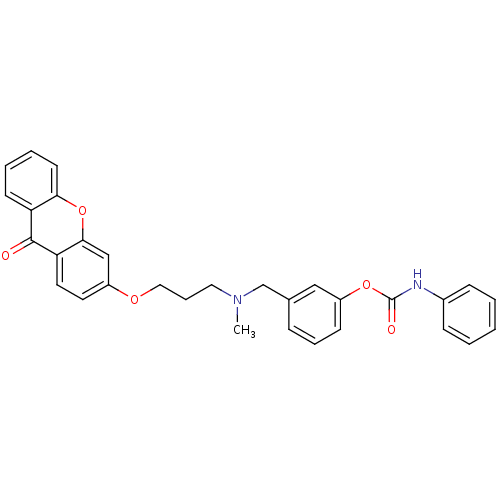
(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CN(CCCOc1ccc2c(c1)oc1ccccc1c2=O)Cc1cccc(OC(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C31H28N2O5/c1-33(21-22-9-7-12-25(19-22)37-31(35)32-23-10-3-2-4-11-23)17-8-18-36-24-15-16-27-29(20-24)38-28-14-6-5-13-26(28)30(27)34/h2-7,9-16,19-20H,8,17-18,21H2,1H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
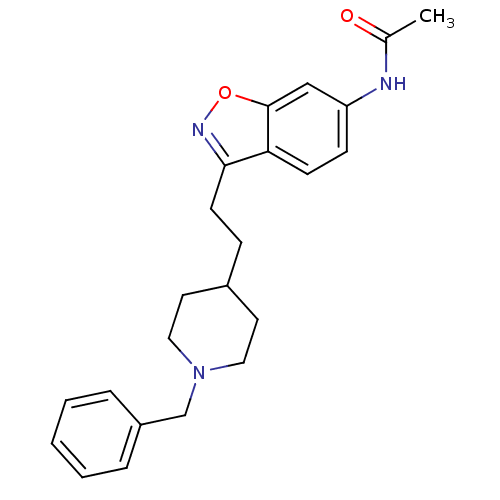
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9028
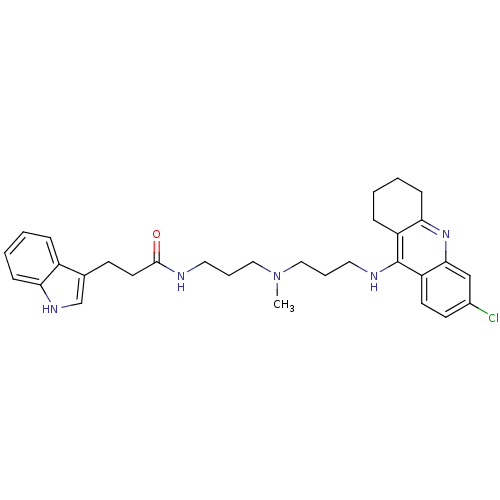
(Indole-Tacrine Heterodimer 11 | N-(3-{[3-(6-Chloro...)Show SMILES CN(CCCNC(=O)CCc1c[nH]c2ccccc12)CCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C31H38ClN5O/c1-37(18-6-16-33-30(38)15-12-22-21-35-27-10-4-2-8-24(22)27)19-7-17-34-31-25-9-3-5-11-28(25)36-29-20-23(32)13-14-26(29)31/h2,4,8,10,13-14,20-21,35H,3,5-7,9,11-12,15-19H2,1H3,(H,33,38)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318726
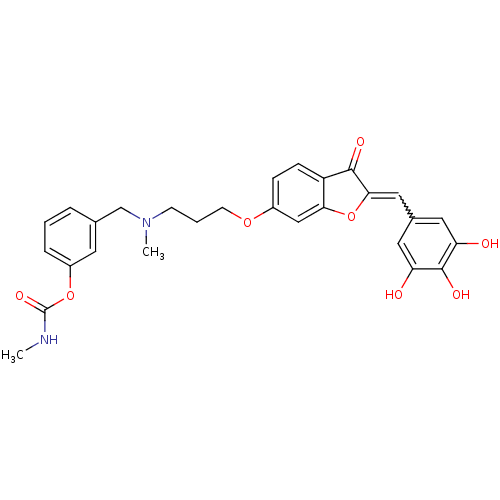
(3-((methyl(3-(3-oxo-2-(3,4,5-trihydroxybenzylidene...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3C(=O)C(Oc3c2)=Cc2cc(O)c(O)c(O)c2)c1 |w:27.29| Show InChI InChI=1S/C28H28N2O8/c1-29-28(35)37-20-6-3-5-17(11-20)16-30(2)9-4-10-36-19-7-8-21-24(15-19)38-25(26(21)33)14-18-12-22(31)27(34)23(32)13-18/h3,5-8,11-15,31-32,34H,4,9-10,16H2,1-2H3,(H,29,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data