

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319300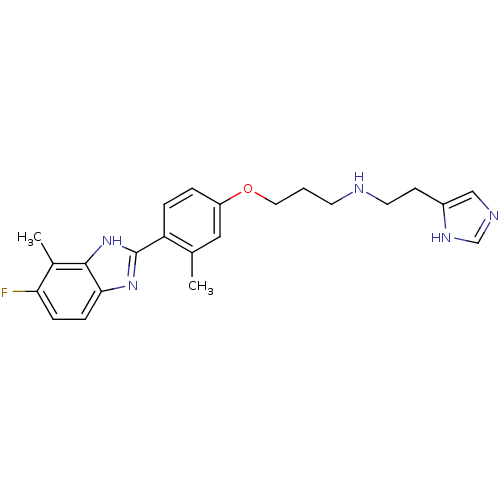 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319302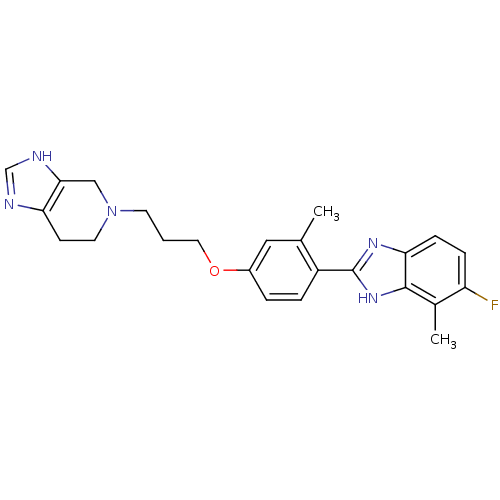 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319295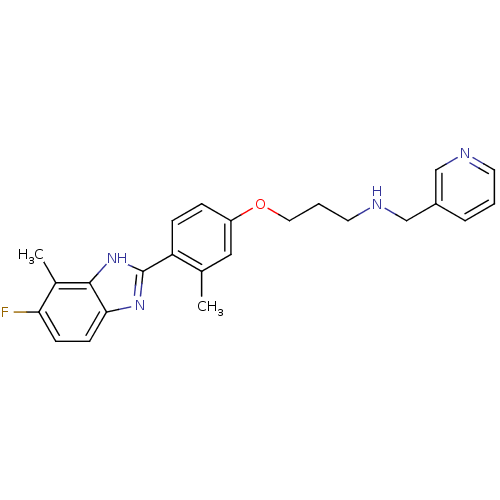 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319285 (5-fluoro-4-methyl-2-(2-methyl-4-(3-((4aR,7aR)-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319279 (1-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22560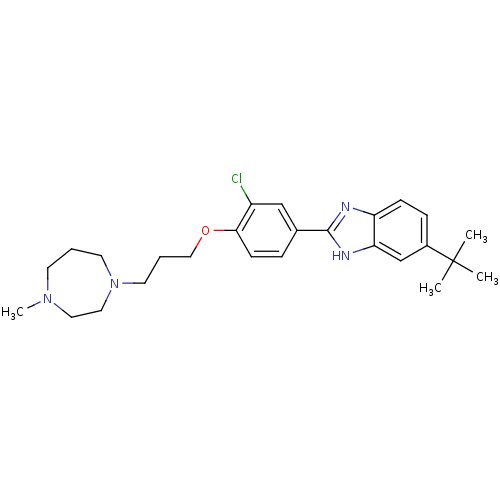 (2-arylbenzimidazole derivative, 6 | 5-tert-butyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319287 ((+/-)-5-fluoro-2-(4-(3-(hexahydropyrrolo[3,4-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319277 (5-fluoro-4-methyl-2-(2-methyl-4-(3-(4-methylpipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319283 ((+/-)-5-fluoro-4-methyl-2-(2-methyl-4-(3-(tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22561 (2-arylbenzimidazole derivative, 7 | 5-tert-butyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319281 (1-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319294 ((+/-)-5-fluoro-4-methyl-2-(2-methyl-4-(3-(3-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319278 (1-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319280 (1-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319293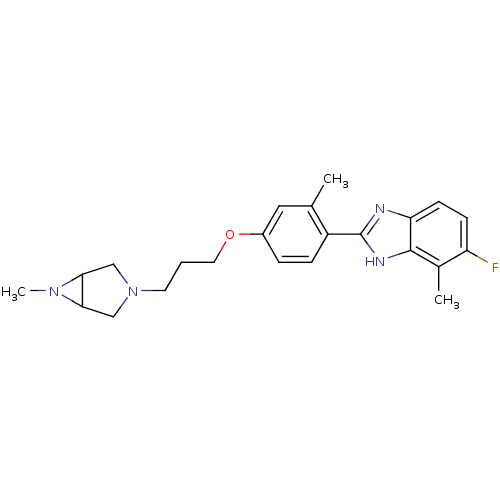 ((+/-)-5-fluoro-4-methyl-2-(2-methyl-4-(3-(6-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319301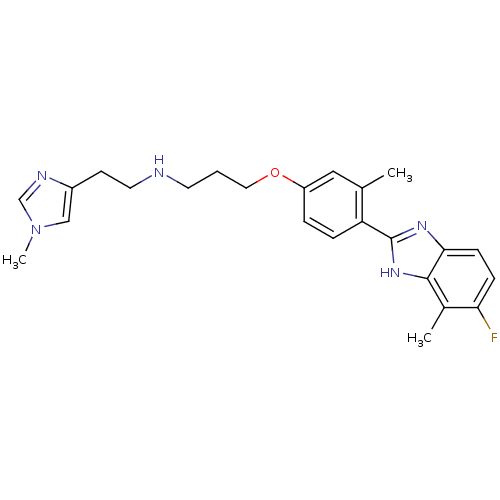 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319303 ((+/-)-3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319297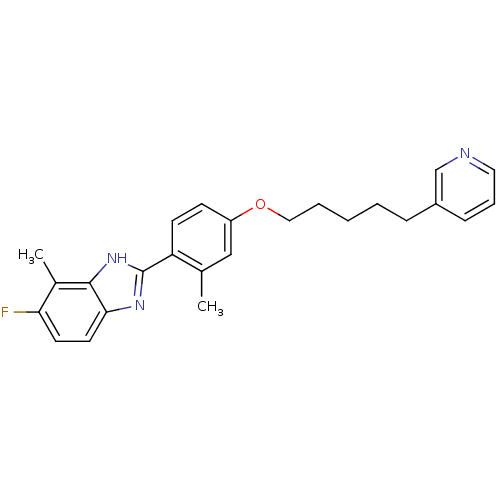 (5-fluoro-4-methyl-2-(2-methyl-4-(5-(pyridin-3-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319298 (2-(4-(3-(5H-pyrrolo[3,4-b]pyridin-6(7H)-yl)propoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319291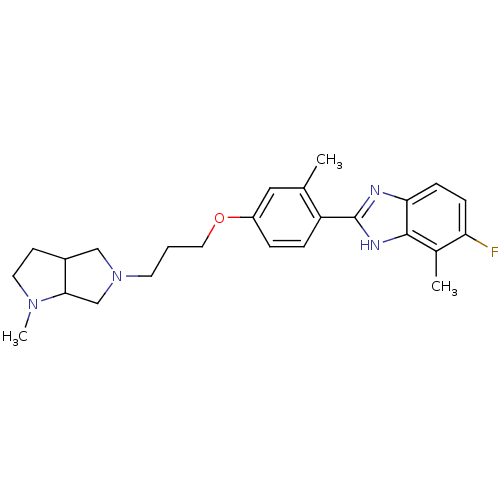 ((+/-)-5-fluoro-4-methyl-2-(2-methyl-4-(3-(1-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22559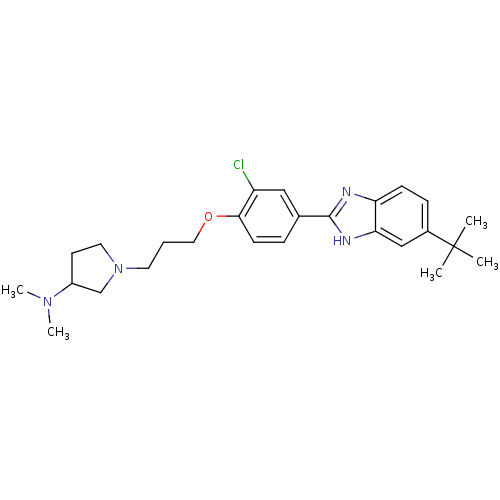 (1-{3-[4-(5-tert-butyl-1H-1,3-benzodiazol-2-yl)-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319308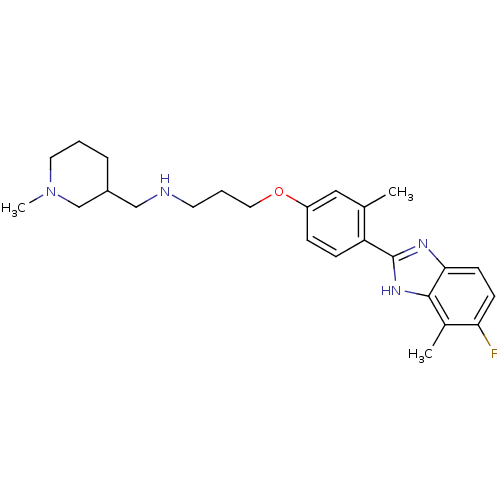 ((+/-)-3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 557 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319305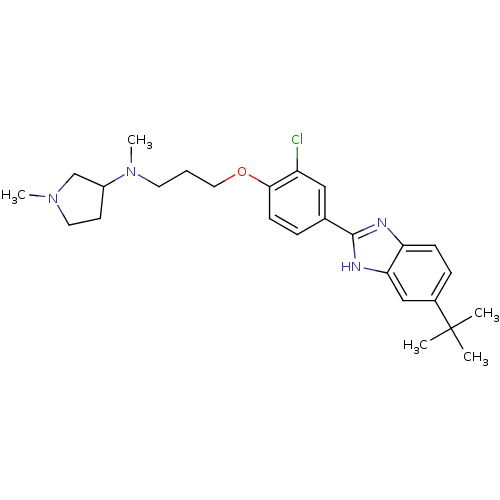 (CHEMBL1085872 | N-(3-(4-(5-tert-butyl-1H-benzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 609 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319286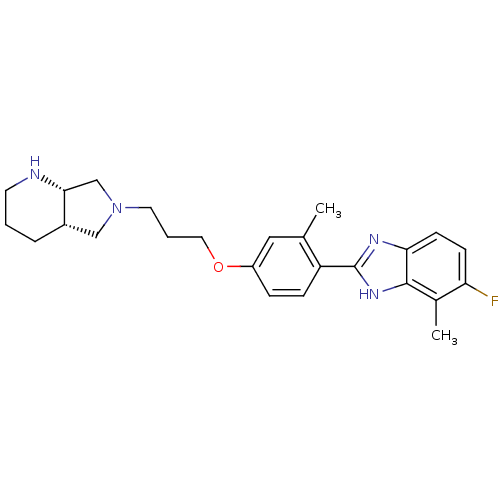 (5-fluoro-4-methyl-2-(2-methyl-4-(3-((4aS,7aS)-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 691 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319292 ((+/-)-5-fluoro-4-methyl-2-(2-methyl-4-(3-(6-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 971 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319284 ((+/-)-5-fluoro-4-methyl-2-(2-methyl-4-(3-(1-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319304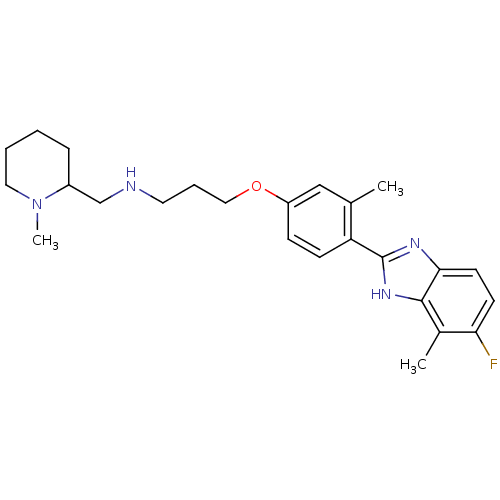 ((+/-)-3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319282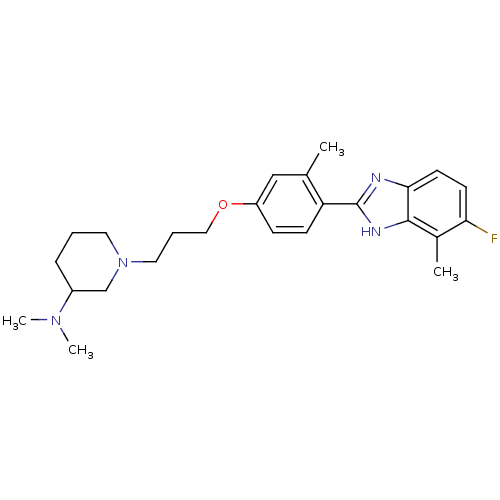 (1-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319306 (CHEMBL1085403 | N1-(3-(4-(5-tert-butyl-1H-inden-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319296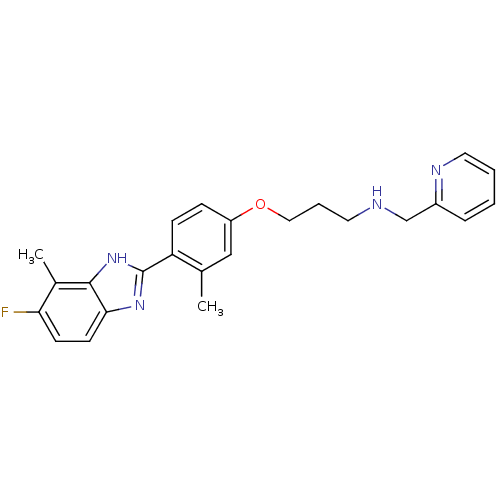 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319288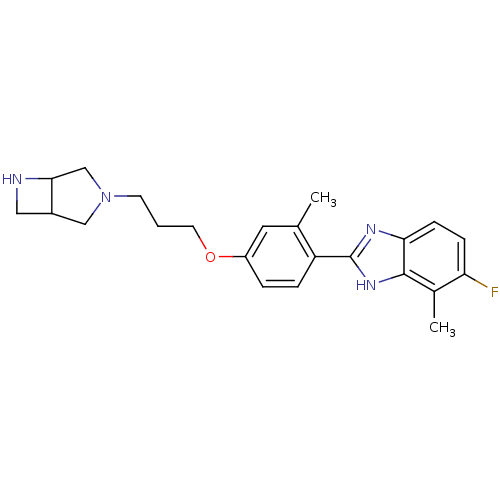 ((+/-)-2-(4-(3-(3,6-diazabicyclo[3.2.0]heptan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319289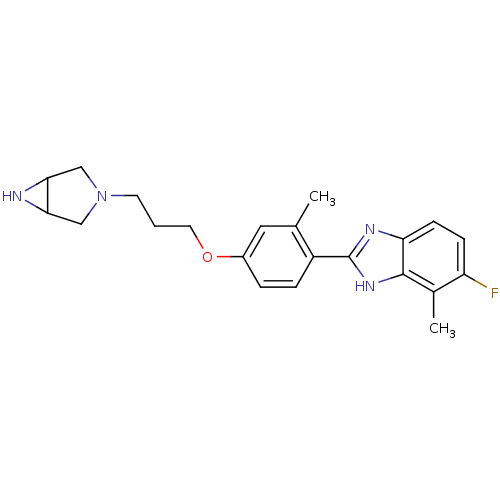 ((+/-)-2-(4-(3-(3,6-diazabicyclo[3.1.0]hexan-3-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319290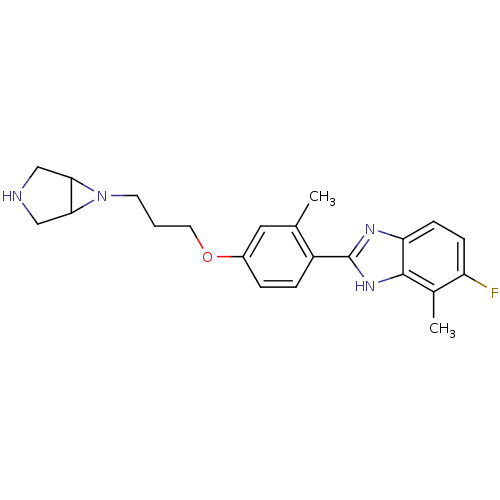 ((+/-)-2-(4-(3-(3,6-diazabicyclo[3.1.0]hexan-6-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319299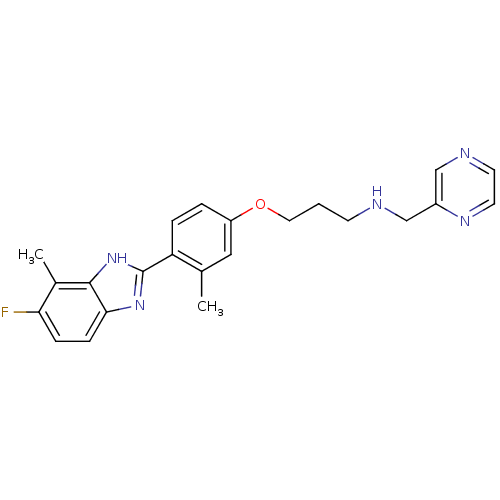 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319302 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319300 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM50319300 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Intrinsic activity at mouse histamine H4 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP responsive elemen... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319307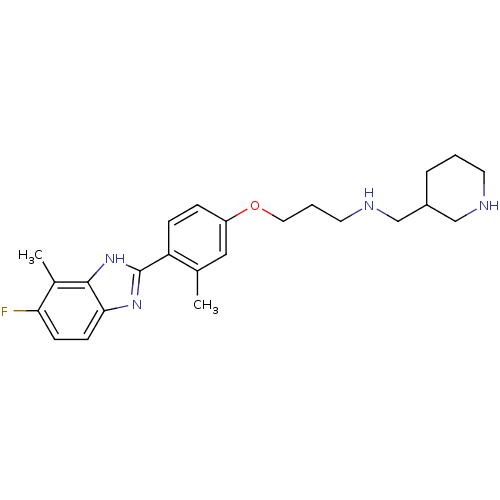 ((+/-)-3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319295 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM50319302 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from mouse recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM50319300 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 548 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from mouse recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM50319285 (5-fluoro-4-methyl-2-(2-methyl-4-(3-((4aR,7aR)-tetr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from mouse recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319302 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 377 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125H]iodoproxyphan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319300 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125H]iodoproxyphan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319285 (5-fluoro-4-methyl-2-(2-methyl-4-(3-((4aR,7aR)-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 119 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125H]iodoproxyphan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50319302 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 131 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50319300 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50319285 (5-fluoro-4-methyl-2-(2-methyl-4-(3-((4aR,7aR)-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50319302 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |