 Found 27 hits Enz. Inhib. hit(s) with all data for entry = 50031893
Found 27 hits Enz. Inhib. hit(s) with all data for entry = 50031893 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321104
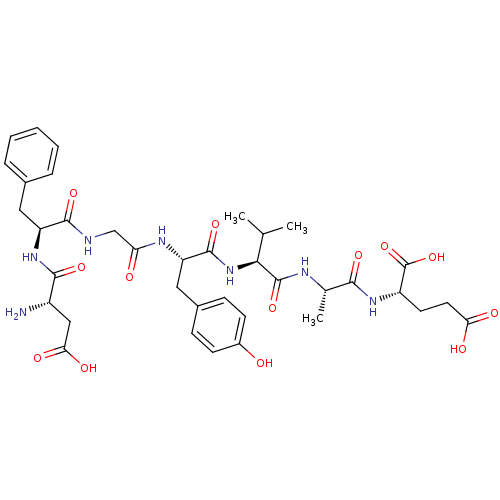
(CHEMBL1163804 | DFGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C37H49N7O13/c1-19(2)31(36(55)40-20(3)32(51)42-25(37(56)57)13-14-29(47)48)44-35(54)27(16-22-9-11-23(45)12-10-22)41-28(46)18-39-34(53)26(15-21-7-5-4-6-8-21)43-33(52)24(38)17-30(49)50/h4-12,19-20,24-27,31,45H,13-18,38H2,1-3H3,(H,39,53)(H,40,55)(H,41,46)(H,42,51)(H,43,52)(H,44,54)(H,47,48)(H,49,50)(H,56,57)/t20-,24-,25-,26-,27-,31-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Competitive inhibition of HMG-CoA reductase by Dixon plot analysis |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321100
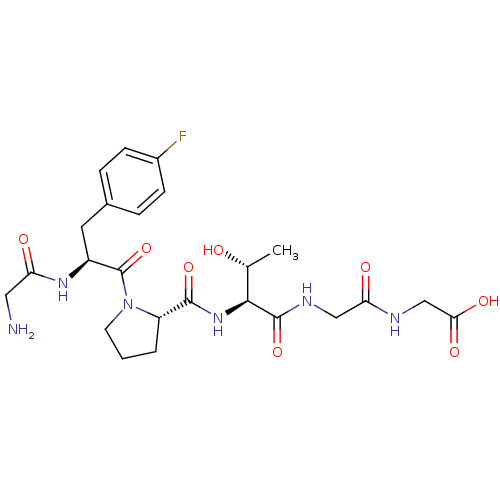
(CHEMBL1164546 | GF(4-fluro)PTGG)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CN)C(=O)NCC(=O)NCC(O)=O |r| Show InChI InChI=1S/C24H33FN6O8/c1-13(32)21(23(38)28-11-19(34)27-12-20(35)36)30-22(37)17-3-2-8-31(17)24(39)16(29-18(33)10-26)9-14-4-6-15(25)7-5-14/h4-7,13,16-17,21,32H,2-3,8-12,26H2,1H3,(H,27,34)(H,28,38)(H,29,33)(H,30,37)(H,35,36)/t13-,16+,17+,21+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Competitive inhibition of HMG-CoA reductase by Dixon plot analysis |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321104

(CHEMBL1163804 | DFGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C37H49N7O13/c1-19(2)31(36(55)40-20(3)32(51)42-25(37(56)57)13-14-29(47)48)44-35(54)27(16-22-9-11-23(45)12-10-22)41-28(46)18-39-34(53)26(15-21-7-5-4-6-8-21)43-33(52)24(38)17-30(49)50/h4-12,19-20,24-27,31,45H,13-18,38H2,1-3H3,(H,39,53)(H,40,55)(H,41,46)(H,42,51)(H,43,52)(H,44,54)(H,47,48)(H,49,50)(H,56,57)/t20-,24-,25-,26-,27-,31-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321109
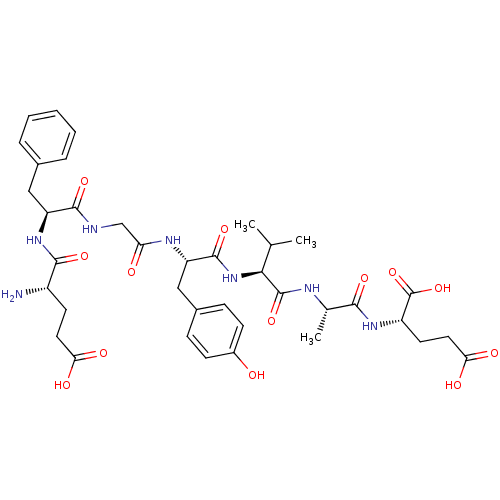
(CHEMBL1163150 | EFGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C38H51N7O13/c1-20(2)32(37(56)41-21(3)33(52)43-26(38(57)58)14-16-31(50)51)45-36(55)28(18-23-9-11-24(46)12-10-23)42-29(47)19-40-35(54)27(17-22-7-5-4-6-8-22)44-34(53)25(39)13-15-30(48)49/h4-12,20-21,25-28,32,46H,13-19,39H2,1-3H3,(H,40,54)(H,41,56)(H,42,47)(H,43,52)(H,44,53)(H,45,55)(H,48,49)(H,50,51)(H,57,58)/t21-,25-,26-,27-,28-,32-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321110
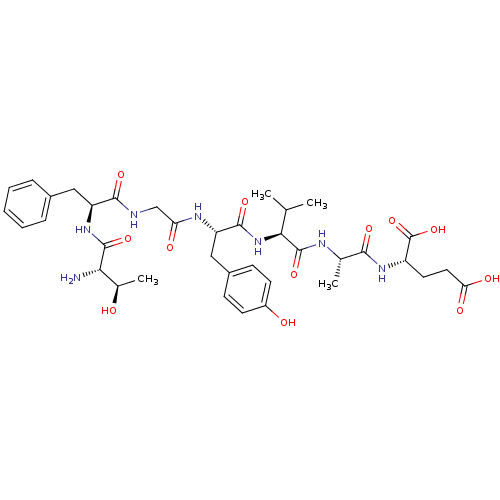
(CHEMBL1165024 | TFGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C37H51N7O12/c1-19(2)31(36(54)40-20(3)32(50)42-25(37(55)56)14-15-29(48)49)44-34(52)27(17-23-10-12-24(46)13-11-23)41-28(47)18-39-33(51)26(16-22-8-6-5-7-9-22)43-35(53)30(38)21(4)45/h5-13,19-21,25-27,30-31,45-46H,14-18,38H2,1-4H3,(H,39,51)(H,40,54)(H,41,47)(H,42,50)(H,43,53)(H,44,52)(H,48,49)(H,55,56)/t20-,21+,25-,26-,27-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321105
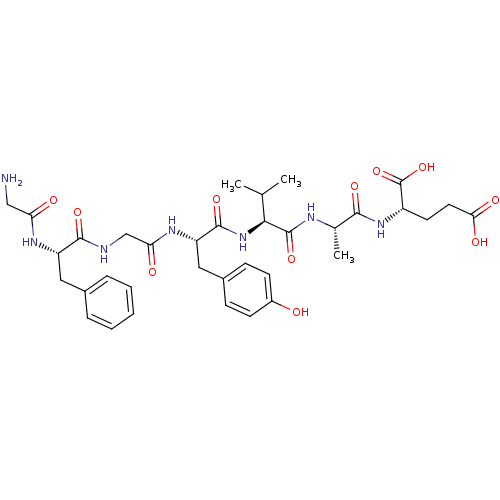
(CHEMBL1163149 | GFGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)CN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C35H47N7O11/c1-19(2)30(34(51)38-20(3)31(48)41-24(35(52)53)13-14-29(46)47)42-33(50)26(16-22-9-11-23(43)12-10-22)40-28(45)18-37-32(49)25(39-27(44)17-36)15-21-7-5-4-6-8-21/h4-12,19-20,24-26,30,43H,13-18,36H2,1-3H3,(H,37,49)(H,38,51)(H,39,44)(H,40,45)(H,41,48)(H,42,50)(H,46,47)(H,52,53)/t20-,24-,25-,26-,30-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321108
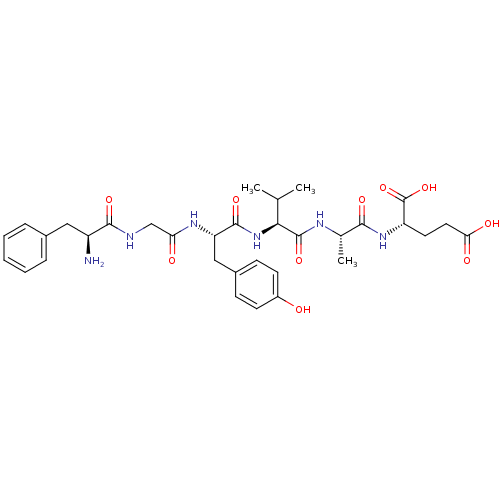
(CHEMBL1163723 | FGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C33H44N6O10/c1-18(2)28(32(47)36-19(3)29(44)38-24(33(48)49)13-14-27(42)43)39-31(46)25(16-21-9-11-22(40)12-10-21)37-26(41)17-35-30(45)23(34)15-20-7-5-4-6-8-20/h4-12,18-19,23-25,28,40H,13-17,34H2,1-3H3,(H,35,45)(H,36,47)(H,37,41)(H,38,44)(H,39,46)(H,42,43)(H,48,49)/t19-,23-,24-,25-,28-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321103

(CHEMBL1165099 | GF(4-fluro)PEGG)Show SMILES NCC(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)NCC(O)=O |r| Show InChI InChI=1S/C25H33FN6O9/c26-15-5-3-14(4-6-15)10-17(30-19(33)11-27)25(41)32-9-1-2-18(32)24(40)31-16(7-8-21(35)36)23(39)29-12-20(34)28-13-22(37)38/h3-6,16-18H,1-2,7-13,27H2,(H,28,34)(H,29,39)(H,30,33)(H,31,40)(H,35,36)(H,37,38)/t16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321107

(CHEMBL1165259 | FPYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C36H48N6O10/c1-20(2)30(34(49)38-21(3)31(46)39-26(36(51)52)15-16-29(44)45)41-32(47)27(19-23-11-13-24(43)14-12-23)40-33(48)28-10-7-17-42(28)35(50)25(37)18-22-8-5-4-6-9-22/h4-6,8-9,11-14,20-21,25-28,30,43H,7,10,15-19,37H2,1-3H3,(H,38,49)(H,39,46)(H,40,48)(H,41,47)(H,44,45)(H,51,52)/t21-,25-,26-,27-,28-,30-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226161
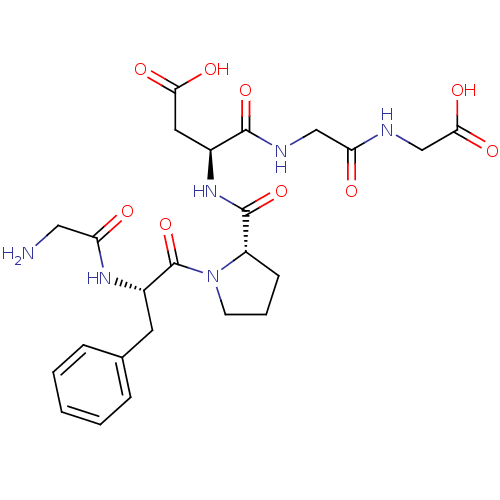
(CHEMBL261622 | GFPDGG)Show SMILES NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)NCC(O)=O Show InChI InChI=1S/C24H32N6O9/c25-11-18(31)28-16(9-14-5-2-1-3-6-14)24(39)30-8-4-7-17(30)23(38)29-15(10-20(33)34)22(37)27-12-19(32)26-13-21(35)36/h1-3,5-6,15-17H,4,7-13,25H2,(H,26,32)(H,27,37)(H,28,31)(H,29,38)(H,33,34)(H,35,36)/t15-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226160
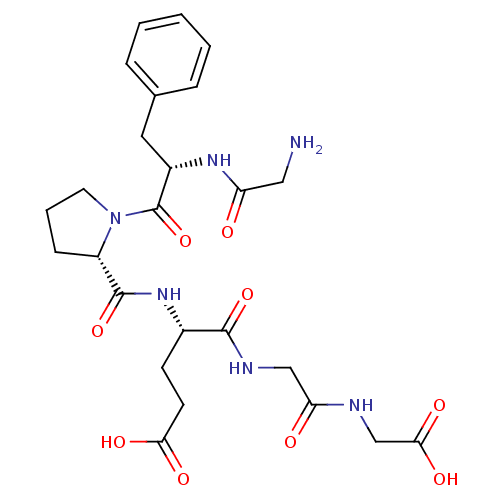
(CHEMBL261621 | GFPEGG)Show SMILES NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)NCC(O)=O Show InChI InChI=1S/C25H34N6O9/c26-12-19(32)29-17(11-15-5-2-1-3-6-15)25(40)31-10-4-7-18(31)24(39)30-16(8-9-21(34)35)23(38)28-13-20(33)27-14-22(36)37/h1-3,5-6,16-18H,4,7-14,26H2,(H,27,33)(H,28,38)(H,29,32)(H,30,39)(H,34,35)(H,36,37)/t16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321101

(CHEMBL1163145 | FFYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C40H50N6O10/c1-23(2)34(39(54)42-24(3)35(50)43-30(40(55)56)18-19-33(48)49)46-38(53)32(22-27-14-16-28(47)17-15-27)45-37(52)31(21-26-12-8-5-9-13-26)44-36(51)29(41)20-25-10-6-4-7-11-25/h4-17,23-24,29-32,34,47H,18-22,41H2,1-3H3,(H,42,54)(H,43,50)(H,44,51)(H,45,52)(H,46,53)(H,48,49)(H,55,56)/t24-,29-,30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226166
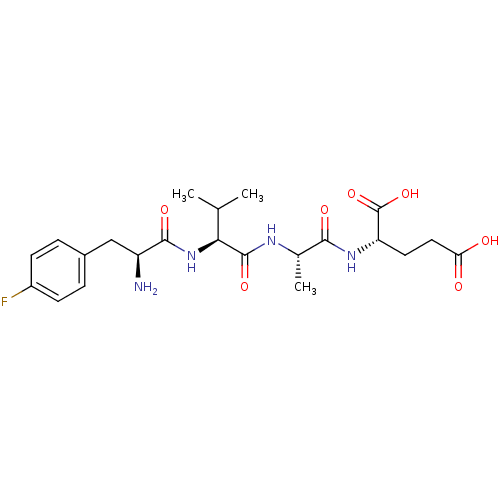
(CHEMBL258583 | F(4-Fluoro)VAE | F(4-fluro)VAE)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)Cc1ccc(F)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H31FN4O7/c1-11(2)18(27-20(31)15(24)10-13-4-6-14(23)7-5-13)21(32)25-12(3)19(30)26-16(22(33)34)8-9-17(28)29/h4-7,11-12,15-16,18H,8-10,24H2,1-3H3,(H,25,32)(H,26,30)(H,27,31)(H,28,29)(H,33,34)/t12-,15-,16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321100

(CHEMBL1164546 | GF(4-fluro)PTGG)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CN)C(=O)NCC(=O)NCC(O)=O |r| Show InChI InChI=1S/C24H33FN6O8/c1-13(32)21(23(38)28-11-19(34)27-12-20(35)36)30-22(37)17-3-2-8-31(17)24(39)16(29-18(33)10-26)9-14-4-6-15(25)7-5-14/h4-7,13,16-17,21,32H,2-3,8-12,26H2,1H3,(H,27,34)(H,28,38)(H,29,33)(H,30,37)(H,35,36)/t13-,16+,17+,21+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321102
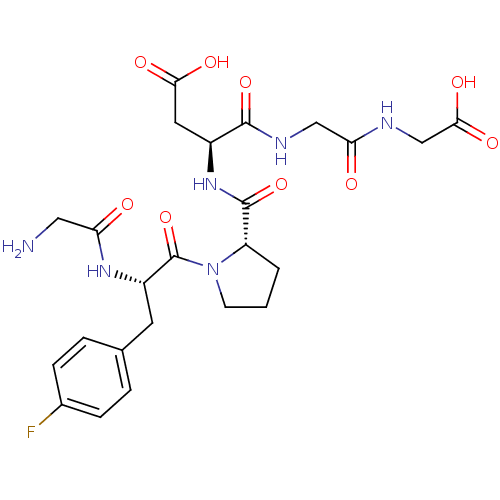
(CHEMBL1164157 | GF(4-fluro)PDGG)Show SMILES NCC(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)NCC(O)=O |r| Show InChI InChI=1S/C24H31FN6O9/c25-14-5-3-13(4-6-14)8-16(29-18(32)10-26)24(40)31-7-1-2-17(31)23(39)30-15(9-20(34)35)22(38)28-11-19(33)27-12-21(36)37/h3-6,15-17H,1-2,7-12,26H2,(H,27,33)(H,28,38)(H,29,32)(H,30,39)(H,34,35)(H,36,37)/t15-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321106

(CHEMBL1163146 | FGF(4-fluro)VAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C33H43FN6O9/c1-18(2)28(32(47)37-19(3)29(44)39-24(33(48)49)13-14-27(42)43)40-31(46)25(16-21-9-11-22(34)12-10-21)38-26(41)17-36-30(45)23(35)15-20-7-5-4-6-8-20/h4-12,18-19,23-25,28H,13-17,35H2,1-3H3,(H,36,45)(H,37,47)(H,38,41)(H,39,44)(H,40,46)(H,42,43)(H,48,49)/t19-,23-,24-,25-,28-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226162
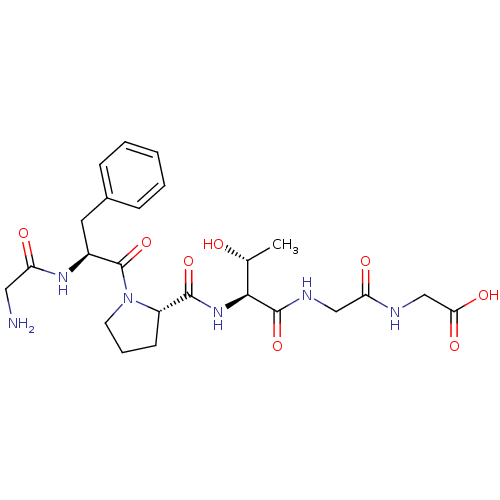
(CHEMBL258999 | GFPTGG)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)CN)C(=O)NCC(=O)NCC(O)=O Show InChI InChI=1S/C24H34N6O8/c1-14(31)21(23(37)27-12-19(33)26-13-20(34)35)29-22(36)17-8-5-9-30(17)24(38)16(28-18(32)11-25)10-15-6-3-2-4-7-15/h2-4,6-7,14,16-17,21,31H,5,8-13,25H2,1H3,(H,26,33)(H,27,37)(H,28,32)(H,29,36)(H,34,35)/t14-,16+,17+,21+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226165

(CHEMBL261334 | GLPTGG)Show SMILES CC(C)C[C@H](NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(O)=O Show InChI InChI=1S/C21H36N6O8/c1-11(2)7-13(25-15(29)8-22)21(35)27-6-4-5-14(27)19(33)26-18(12(3)28)20(34)24-9-16(30)23-10-17(31)32/h11-14,18,28H,4-10,22H2,1-3H3,(H,23,30)(H,24,34)(H,25,29)(H,26,33)(H,31,32)/t12-,13+,14+,18+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226164

(CHEMBL412295 | GLPDGG)Show SMILES CC(C)C[C@H](NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)NCC(O)=O Show InChI InChI=1S/C21H34N6O9/c1-11(2)6-13(25-15(28)8-22)21(36)27-5-3-4-14(27)20(35)26-12(7-17(30)31)19(34)24-9-16(29)23-10-18(32)33/h11-14H,3-10,22H2,1-2H3,(H,23,29)(H,24,34)(H,25,28)(H,26,35)(H,30,31)(H,32,33)/t12-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226163

(CHEMBL410639 | GLPEGG)Show SMILES CC(C)C[C@H](NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)NCC(O)=O Show InChI InChI=1S/C22H36N6O9/c1-12(2)8-14(26-16(29)9-23)22(37)28-7-3-4-15(28)21(36)27-13(5-6-18(31)32)20(35)25-10-17(30)24-11-19(33)34/h12-15H,3-11,23H2,1-2H3,(H,24,30)(H,25,35)(H,26,29)(H,27,36)(H,31,32)(H,33,34)/t13-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226168

(CHEMBL411443 | YVAE)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H32N4O8/c1-11(2)18(26-20(31)15(23)10-13-4-6-14(27)7-5-13)21(32)24-12(3)19(30)25-16(22(33)34)8-9-17(28)29/h4-7,11-12,15-16,18,27H,8-10,23H2,1-3H3,(H,24,32)(H,25,30)(H,26,31)(H,28,29)(H,33,34)/t12-,15-,16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226170
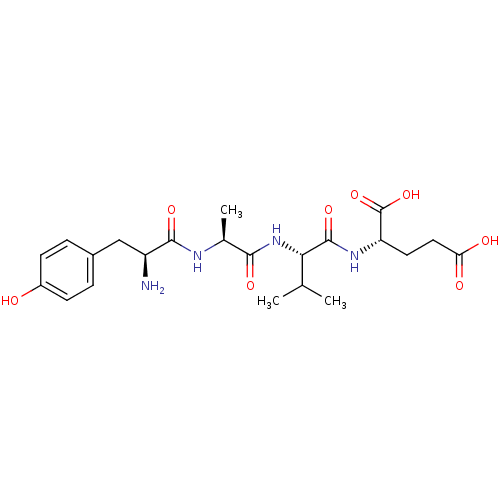
(CHEMBL409841 | YAVE)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H32N4O8/c1-11(2)18(21(32)25-16(22(33)34)8-9-17(28)29)26-19(30)12(3)24-20(31)15(23)10-13-4-6-14(27)7-5-13/h4-7,11-12,15-16,18,27H,8-10,23H2,1-3H3,(H,24,31)(H,25,32)(H,26,30)(H,28,29)(H,33,34)/t12-,15-,16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226169

(CHEMBL261748 | IVAE)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C19H34N4O7/c1-6-10(4)14(20)17(27)23-15(9(2)3)18(28)21-11(5)16(26)22-12(19(29)30)7-8-13(24)25/h9-12,14-15H,6-8,20H2,1-5H3,(H,21,28)(H,22,26)(H,23,27)(H,24,25)(H,29,30)/t10-,11-,12-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226171
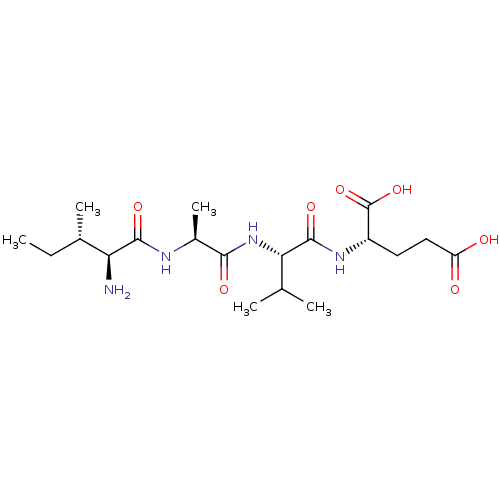
(CHEMBL259240 | IAVE)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C19H34N4O7/c1-6-10(4)14(20)17(27)21-11(5)16(26)23-15(9(2)3)18(28)22-12(19(29)30)7-8-13(24)25/h9-12,14-15H,6-8,20H2,1-5H3,(H,21,27)(H,22,28)(H,23,26)(H,24,25)(H,29,30)/t10-,11-,12-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321111
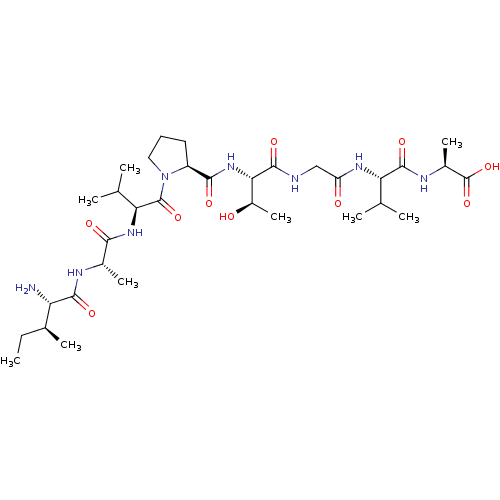
(CHEMBL1163198 | IAVPTGVA)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C33H58N8O10/c1-10-17(6)23(34)29(46)36-18(7)27(44)39-25(16(4)5)32(49)41-13-11-12-21(41)28(45)40-26(20(9)42)30(47)35-14-22(43)38-24(15(2)3)31(48)37-19(8)33(50)51/h15-21,23-26,42H,10-14,34H2,1-9H3,(H,35,47)(H,36,46)(H,37,48)(H,38,43)(H,39,44)(H,40,45)(H,50,51)/t17-,18-,19-,20+,21-,23-,24-,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226173

(CHEMBL260510 | IAVPGEVA)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O Show InChI InChI=1S/C34H58N8O11/c1-9-18(6)25(35)31(49)37-19(7)28(46)41-27(17(4)5)33(51)42-14-10-11-22(42)30(48)36-15-23(43)39-21(12-13-24(44)45)29(47)40-26(16(2)3)32(50)38-20(8)34(52)53/h16-22,25-27H,9-15,35H2,1-8H3,(H,36,48)(H,37,49)(H,38,50)(H,39,43)(H,40,47)(H,41,46)(H,44,45)(H,52,53)/t18-,19-,20-,21-,22-,25-,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50226159

(CHEMBL412060 | LPYP)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C25H36N4O6/c1-15(2)13-18(26)23(32)28-11-3-5-20(28)22(31)27-19(14-16-7-9-17(30)10-8-16)24(33)29-12-4-6-21(29)25(34)35/h7-10,15,18-21,30H,3-6,11-14,26H2,1-2H3,(H,27,31)(H,34,35)/t18-,19-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase by spectrophotometry |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data