

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50000041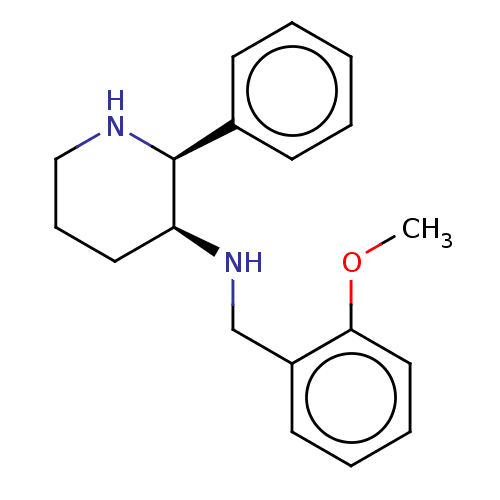 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097740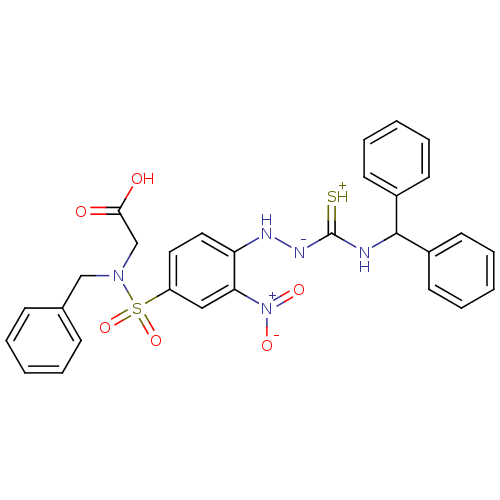 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097732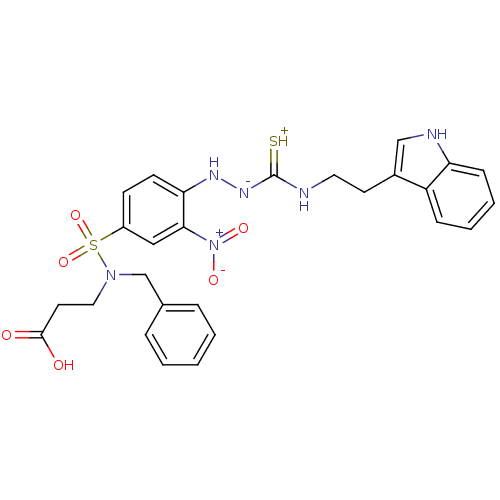 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50097727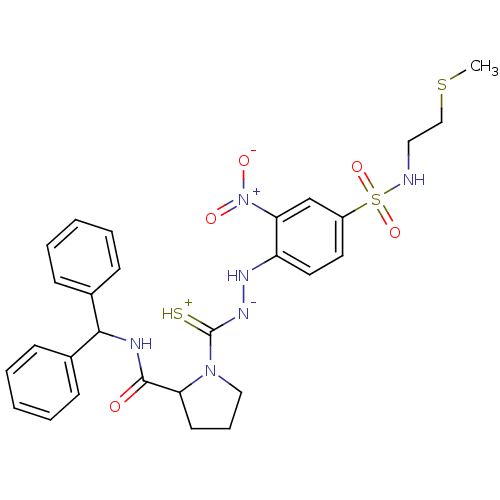 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50097733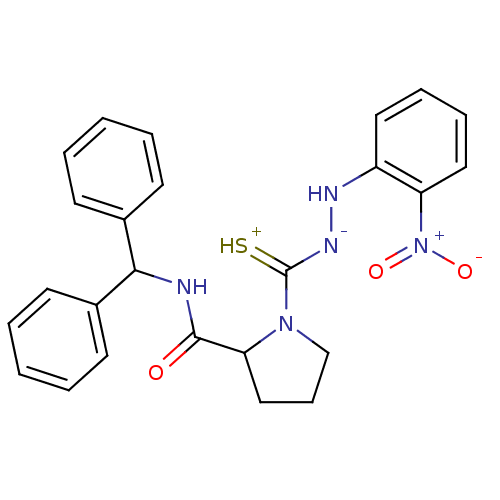 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097726 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097740 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097730 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097742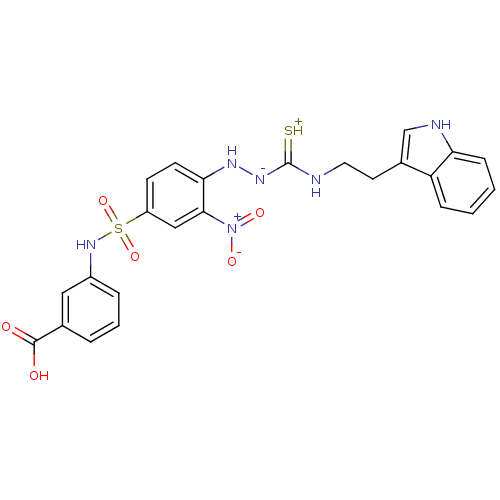 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097743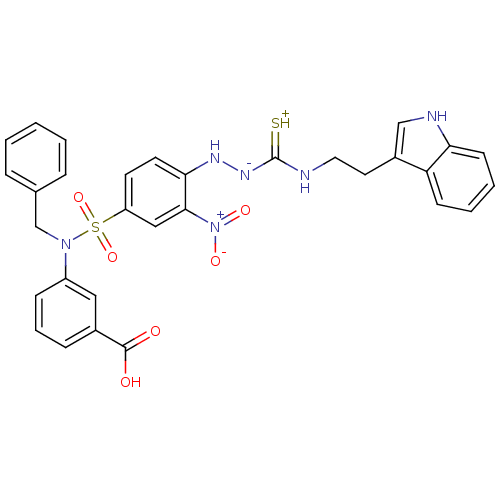 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097738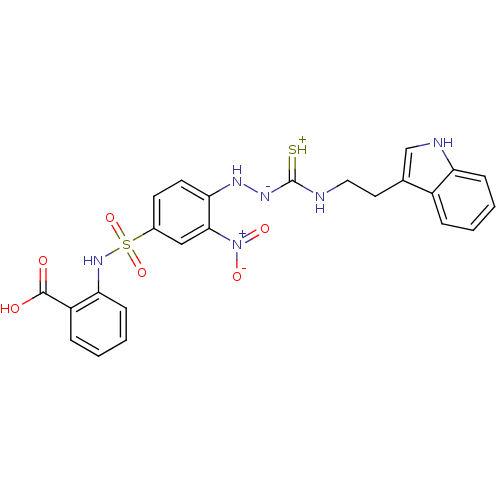 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097731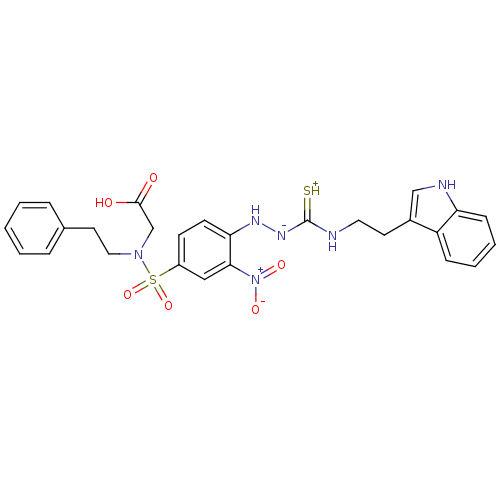 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097725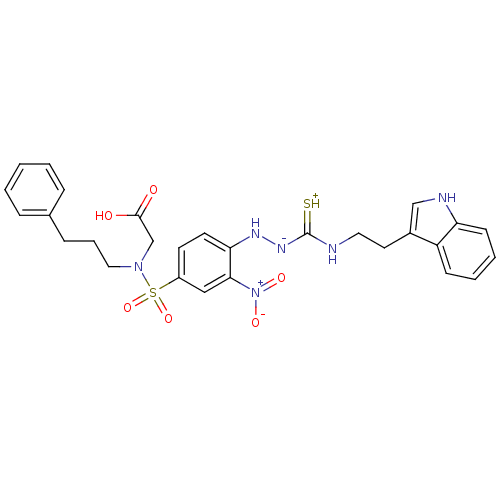 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50097727 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097727 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in guinea pig ileum using organ bath assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097728 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097723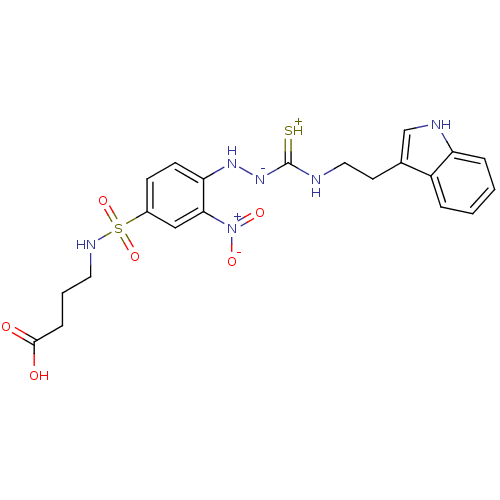 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097729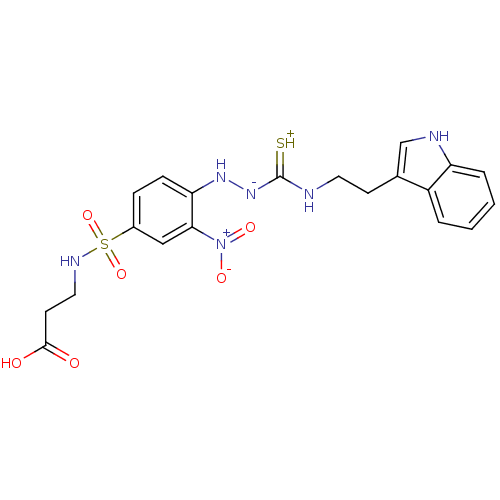 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097734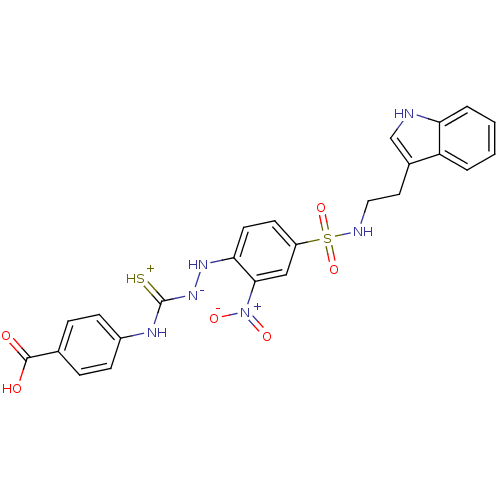 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097737 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50097736 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Cholecystokinin type B receptor in mouse cerebral cortical membranes using [125I]Tyr(SO3H)27]-CCK-8 binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097727 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rabbit whole brain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50097724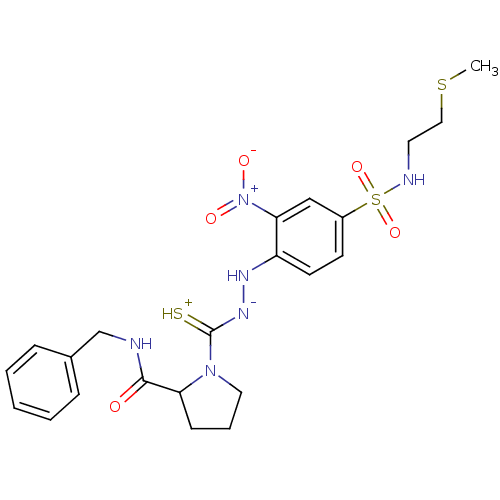 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rat whole forebrain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097724 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rabbit whole brain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097724 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rat whole forebrain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097743 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50097724 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rat whole forebrain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097731 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097738 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097726 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097742 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097724 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rabbit whole brain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097734 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097723 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Tachykinin receptor 1 in rat whole forebrain membranes using [3H][Sar9Met(O2)]-SP binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097730 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097737 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097732 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097727 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097736 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097728 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097729 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097725 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097727 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |