 Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50031984
Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50031984 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50322767
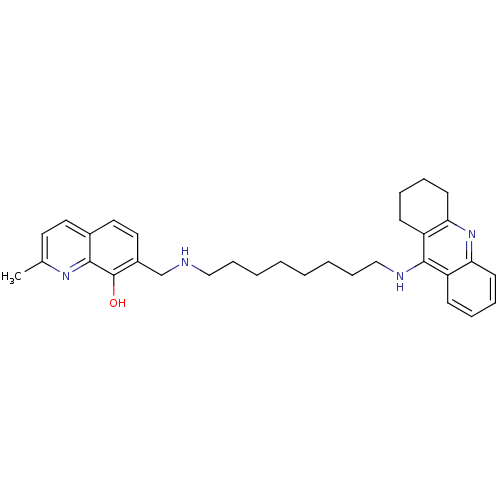
(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50322768
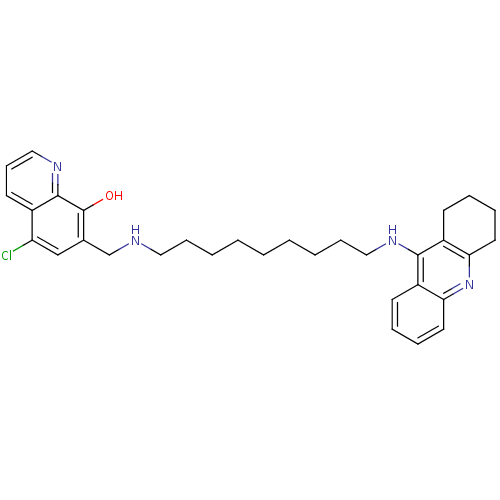
(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322767

(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322766
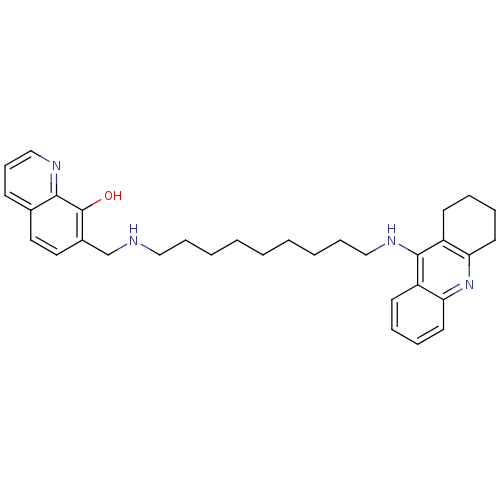
(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50322766

(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322768

(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50322767

(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322777
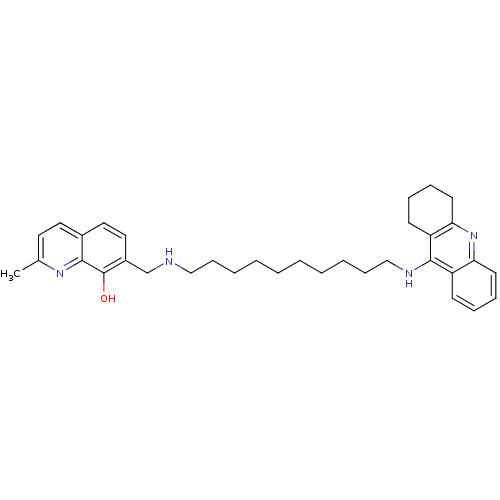
(2-Methyl-7-{[10-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C34H44N4O/c1-25-18-19-26-20-21-27(34(39)32(26)37-25)24-35-22-12-6-4-2-3-5-7-13-23-36-33-28-14-8-10-16-30(28)38-31-17-11-9-15-29(31)33/h8,10,14,16,18-21,35,39H,2-7,9,11-13,15,17,22-24H2,1H3,(H,36,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50322766

(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322766

(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322770
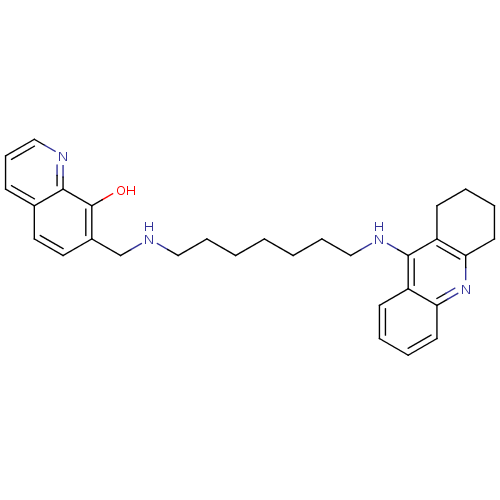
(7-{[7-(1,2,3,4-Tetrahydroacridin-9-ylamino)heptyla...)Show SMILES Oc1c(CNCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C30H36N4O/c35-30-23(17-16-22-11-10-20-32-28(22)30)21-31-18-8-2-1-3-9-19-33-29-24-12-4-6-14-26(24)34-27-15-7-5-13-25(27)29/h4,6,10-12,14,16-17,20,31,35H,1-3,5,7-9,13,15,18-19,21H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322782
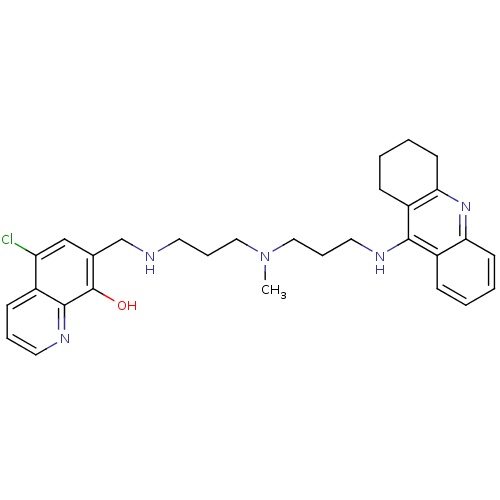
(5-Chloro-7-{{3-{methyl[3-(1,2,3,4-tetrahydroacridi...)Show SMILES CN(CCCNCc1cc(Cl)c2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H36ClN5O/c1-36(17-7-14-32-20-21-19-25(31)22-11-6-15-34-29(22)30(21)37)18-8-16-33-28-23-9-2-4-12-26(23)35-27-13-5-3-10-24(27)28/h2,4,6,9,11-12,15,19,32,37H,3,5,7-8,10,13-14,16-18,20H2,1H3,(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322771
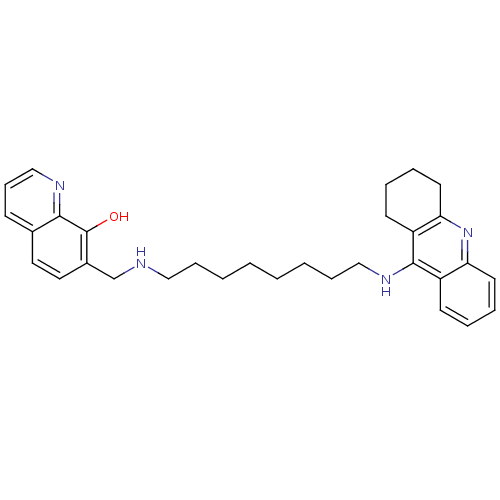
(7-{[8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show SMILES Oc1c(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C31H38N4O/c36-31-24(18-17-23-12-11-21-33-29(23)31)22-32-19-9-3-1-2-4-10-20-34-30-25-13-5-7-15-27(25)35-28-16-8-6-14-26(28)30/h5,7,11-13,15,17-18,21,32,36H,1-4,6,8-10,14,16,19-20,22H2,(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322772
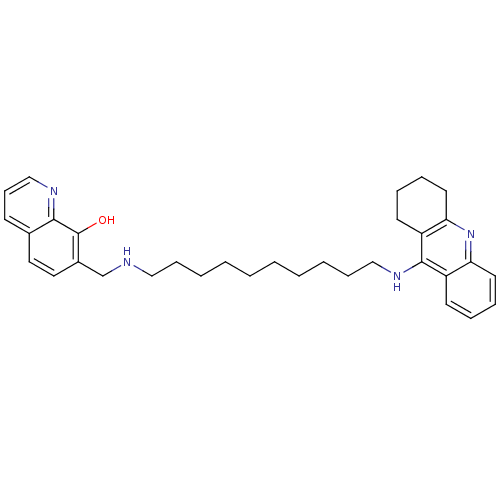
(7-{[10-(1,2,3,4-Tetrahydroacridin-9-ylamino)decyla...)Show SMILES Oc1c(CNCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C33H42N4O/c38-33-26(20-19-25-14-13-23-35-31(25)33)24-34-21-11-5-3-1-2-4-6-12-22-36-32-27-15-7-9-17-29(27)37-30-18-10-8-16-28(30)32/h7,9,13-15,17,19-20,23,34,38H,1-6,8,10-12,16,18,21-22,24H2,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322774
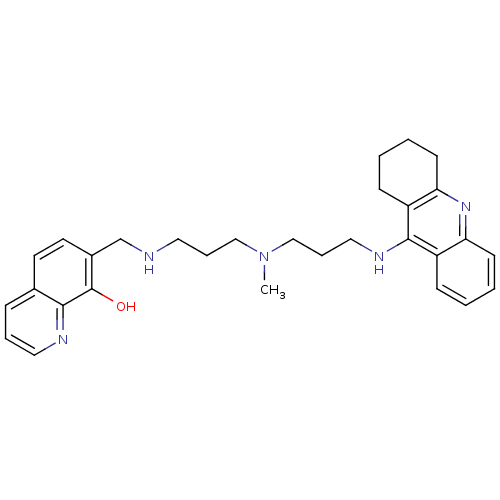
(7-{{3-{Methyl[3-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES CN(CCCNCc1ccc2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N5O/c1-35(19-7-16-31-21-23-15-14-22-9-6-17-32-28(22)30(23)36)20-8-18-33-29-24-10-2-4-12-26(24)34-27-13-5-3-11-25(27)29/h2,4,6,9-10,12,14-15,17,31,36H,3,5,7-8,11,13,16,18-21H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50322768

(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322772

(7-{[10-(1,2,3,4-Tetrahydroacridin-9-ylamino)decyla...)Show SMILES Oc1c(CNCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C33H42N4O/c38-33-26(20-19-25-14-13-23-35-31(25)33)24-34-21-11-5-3-1-2-4-6-12-22-36-32-27-15-7-9-17-29(27)37-30-18-10-8-16-28(30)32/h7,9,13-15,17,19-20,23,34,38H,1-6,8,10-12,16,18,21-22,24H2,(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322777

(2-Methyl-7-{[10-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C34H44N4O/c1-25-18-19-26-20-21-27(34(39)32(26)37-25)24-35-22-12-6-4-2-3-5-7-13-23-36-33-28-14-8-10-16-30(28)38-31-17-11-9-15-29(31)33/h8,10,14,16,18-21,35,39H,2-7,9,11-13,15,17,22-24H2,1H3,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322767

(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322774

(7-{{3-{Methyl[3-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES CN(CCCNCc1ccc2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N5O/c1-35(19-7-16-31-21-23-15-14-22-9-6-17-32-28(22)30(23)36)20-8-18-33-29-24-10-2-4-12-26(24)34-27-13-5-3-11-25(27)29/h2,4,6,9-10,12,14-15,17,31,36H,3,5,7-8,11,13,16,18-21H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322768

(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322771

(7-{[8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show SMILES Oc1c(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C31H38N4O/c36-31-24(18-17-23-12-11-21-33-29(23)31)22-32-19-9-3-1-2-4-10-20-34-30-25-13-5-7-15-27(25)35-28-16-8-6-14-26(28)30/h5,7,11-13,15,17-18,21,32,36H,1-4,6,8-10,14,16,19-20,22H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322782

(5-Chloro-7-{{3-{methyl[3-(1,2,3,4-tetrahydroacridi...)Show SMILES CN(CCCNCc1cc(Cl)c2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H36ClN5O/c1-36(17-7-14-32-20-21-19-25(31)22-11-6-15-34-29(22)30(21)37)18-8-16-33-28-23-9-2-4-12-26(23)35-27-13-5-3-10-24(27)28/h2,4,6,9,11-12,15,19,32,37H,3,5,7-8,10,13-14,16-18,20H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322776
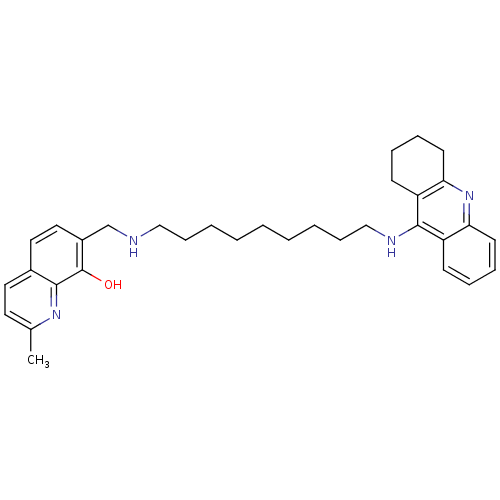
(2-Methyl-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C33H42N4O/c1-24-17-18-25-19-20-26(33(38)31(25)36-24)23-34-21-11-5-3-2-4-6-12-22-35-32-27-13-7-9-15-29(27)37-30-16-10-8-14-28(30)32/h7,9,13,15,17-20,34,38H,2-6,8,10-12,14,16,21-23H2,1H3,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322780

(5-Chloro-7-{[10-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES Oc1c(CNCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C33H41ClN4O/c34-28-22-24(33(39)32-25(28)16-13-21-37-32)23-35-19-11-5-3-1-2-4-6-12-20-36-31-26-14-7-9-17-29(26)38-30-18-10-8-15-27(30)31/h7,9,13-14,16-17,21-22,35,39H,1-6,8,10-12,15,18-20,23H2,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM32203

(8-quinolinol | CHEMBL310555 | US10005735, Table 1....)Show InChI InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322770

(7-{[7-(1,2,3,4-Tetrahydroacridin-9-ylamino)heptyla...)Show SMILES Oc1c(CNCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C30H36N4O/c35-30-23(17-16-22-11-10-20-32-28(22)30)21-31-18-8-2-1-3-9-19-33-29-24-12-4-6-14-26(24)34-27-15-7-5-13-25(27)29/h4,6,10-12,14,16-17,20,31,35H,1-3,5,7-9,13,15,18-19,21H2,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322779

(5-Chloro-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C31H37ClN4O/c32-26-20-22(31(37)30-23(26)14-11-19-35-30)21-33-17-9-3-1-2-4-10-18-34-29-24-12-5-7-15-27(24)36-28-16-8-6-13-25(28)29/h5,7,11-12,14-15,19-20,33,37H,1-4,6,8-10,13,16-18,21H2,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322769

(7-{[6-(1,2,3,4-Tetrahydroacridin-9-ylamino)hexylam...)Show SMILES Oc1c(CNCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C29H34N4O/c34-29-22(16-15-21-10-9-19-31-27(21)29)20-30-17-7-1-2-8-18-32-28-23-11-3-5-13-25(23)33-26-14-6-4-12-24(26)28/h3,5,9-11,13,15-16,19,30,34H,1-2,4,6-8,12,14,17-18,20H2,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322778

(2-Methyl-7-{{3-{methyl[3-(1,2,3,4-tetrahydroacridi...)Show SMILES CN(CCCNCc1ccc2ccc(C)nc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H39N5O/c1-22-13-14-23-15-16-24(31(37)29(23)34-22)21-32-17-7-19-36(2)20-8-18-33-30-25-9-3-5-11-27(25)35-28-12-6-4-10-26(28)30/h3,5,9,11,13-16,32,37H,4,6-8,10,12,17-21H2,1-2H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322775
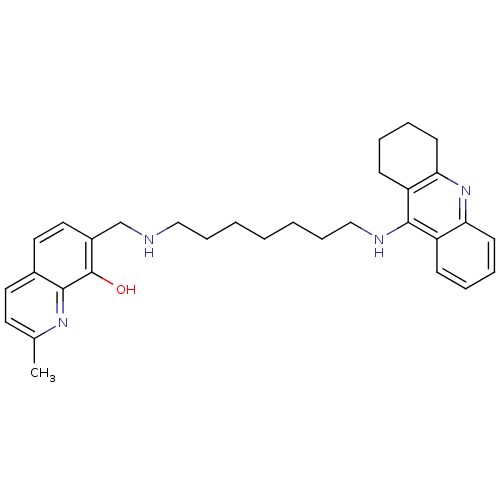
(2-Methyl-7-{[7-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C31H38N4O/c1-22-15-16-23-17-18-24(31(36)29(23)34-22)21-32-19-9-3-2-4-10-20-33-30-25-11-5-7-13-27(25)35-28-14-8-6-12-26(28)30/h5,7,11,13,15-18,32,36H,2-4,6,8-10,12,14,19-21H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322773

(7-{[12-(1,2,3,4-Tetrahydroacridin-9-ylamino)dodecy...)Show SMILES Oc1c(CNCCCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C35H46N4O/c40-35-28(22-21-27-16-15-25-37-33(27)35)26-36-23-13-7-5-3-1-2-4-6-8-14-24-38-34-29-17-9-11-19-31(29)39-32-20-12-10-18-30(32)34/h9,11,15-17,19,21-22,25,36,40H,1-8,10,12-14,18,20,23-24,26H2,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322781

(5-Chloro-7-{[12-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES Oc1c(CNCCCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C35H45ClN4O/c36-30-24-26(35(41)34-27(30)18-15-23-39-34)25-37-21-13-7-5-3-1-2-4-6-8-14-22-38-33-28-16-9-11-19-31(28)40-32-20-12-10-17-29(32)33/h9,11,15-16,18-19,23-24,37,41H,1-8,10,12-14,17,20-22,25H2,(H,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data