

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50200841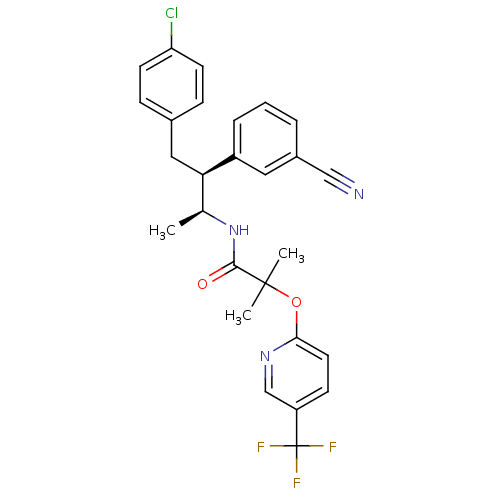 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325211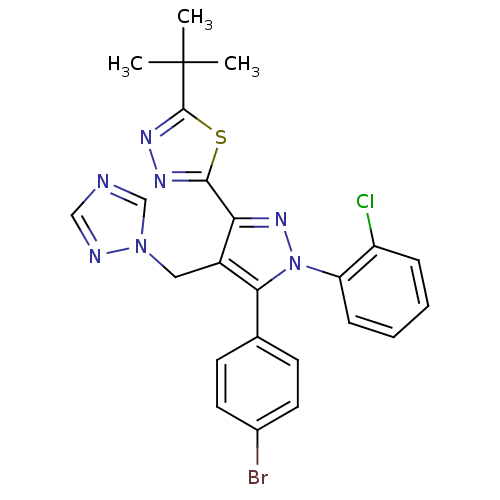 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.681 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325221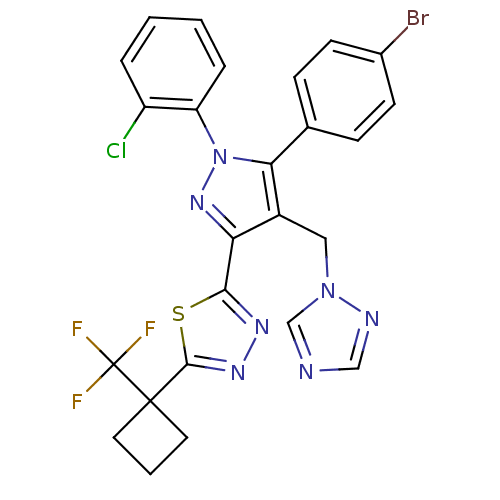 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.848 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325226 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.877 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325223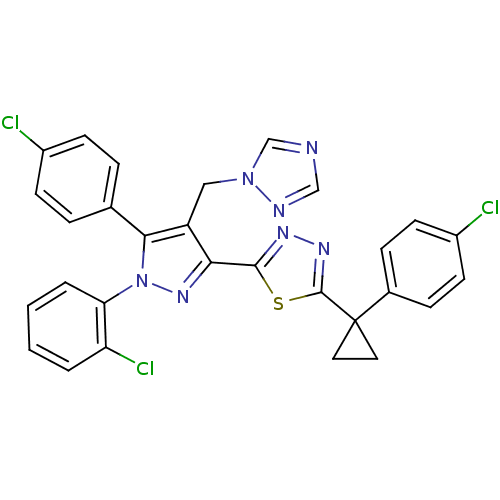 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-1-(2-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.966 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325227 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.991 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325222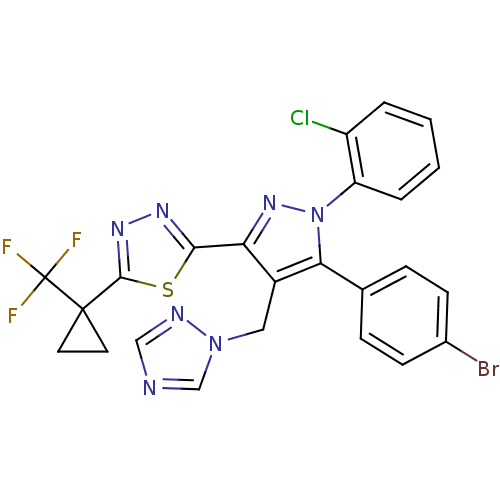 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.992 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325228 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-1-(2-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325213 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325229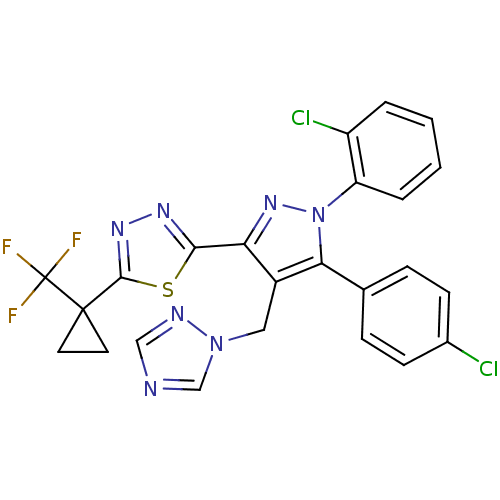 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-1-(2-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325224 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325230 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325231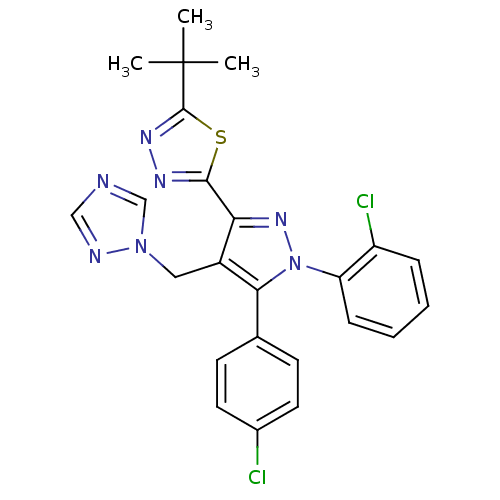 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-1-(2-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325232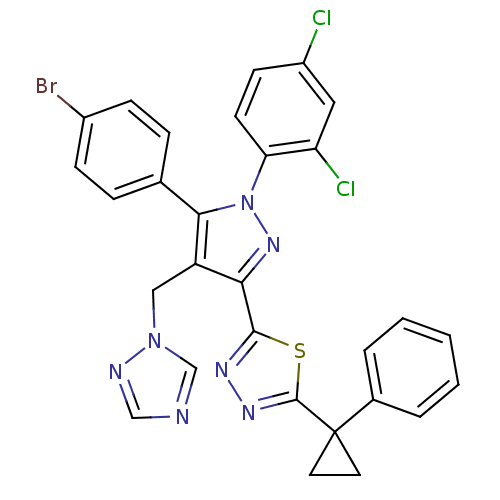 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325225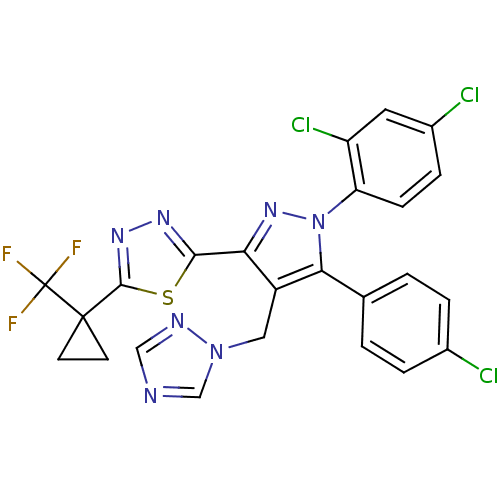 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325233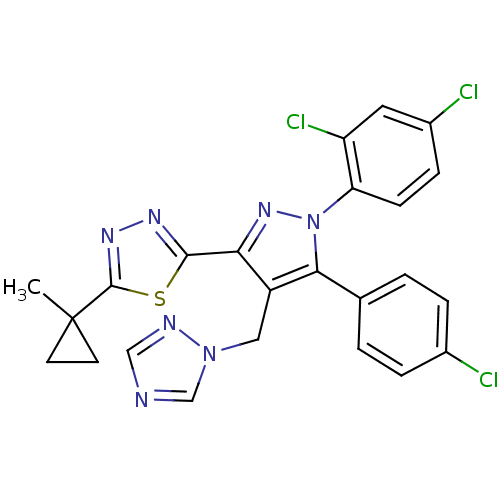 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325214 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50325213 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50325213 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325215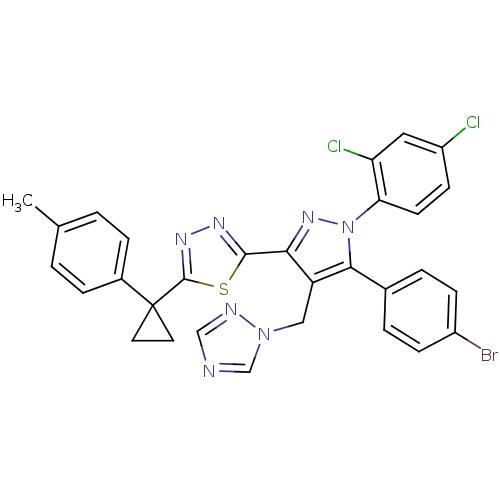 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325212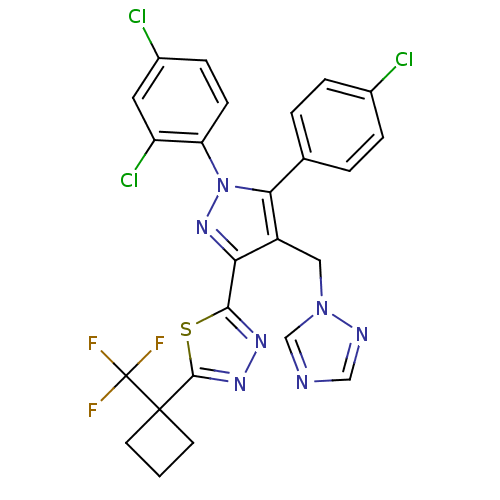 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325216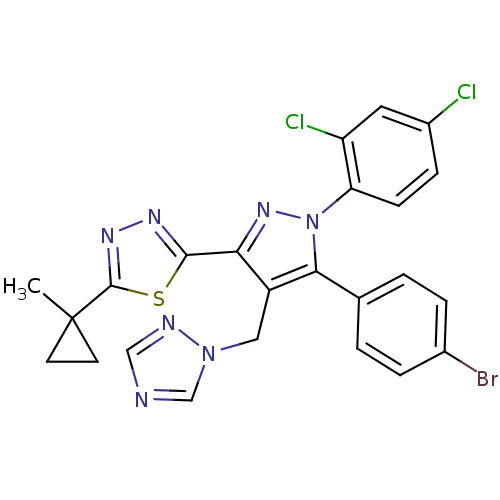 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325217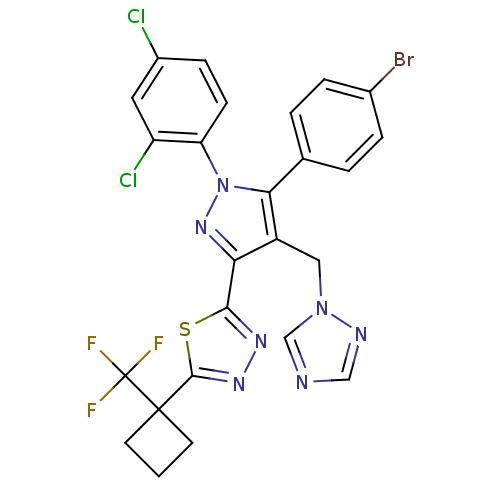 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325218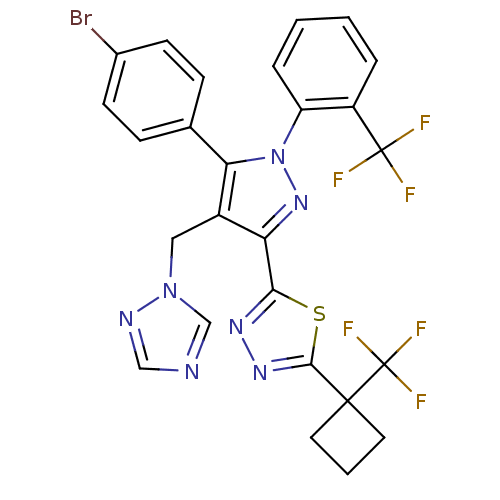 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325219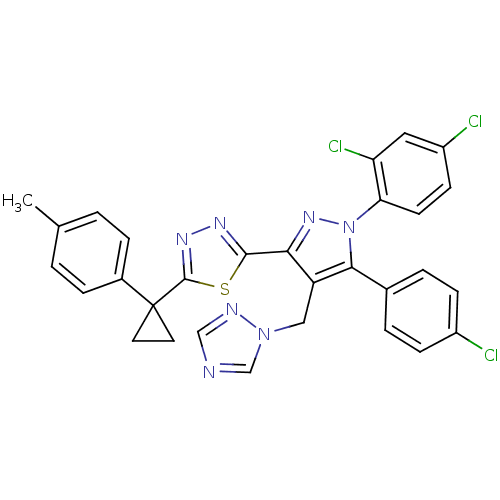 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50325220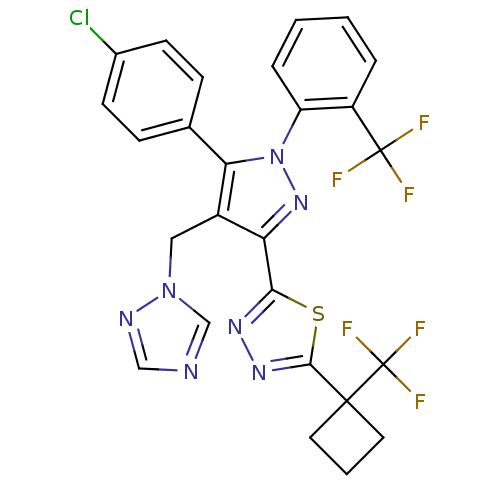 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat cerebellum after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50325211 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50325212 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50325211 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50325211 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50325212 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50325212 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50325213 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50325212 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50325213 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50325211 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes after 30 mins | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50325218 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]WIN-55212-2 from human CB2 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50325221 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]WIN-55212-2 from human CB2 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50325220 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]WIN-55212-2 from human CB2 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50325222 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]WIN-55212-2 from human CB2 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50325223 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-1-(2-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]WIN-55212-2 from human CB2 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50325213 (2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]WIN-55212-2 from human CB2 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 6377-88 (2010) Article DOI: 10.1016/j.bmc.2010.07.013 BindingDB Entry DOI: 10.7270/Q2XG9RBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |