 Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50032253
Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50032253 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326249
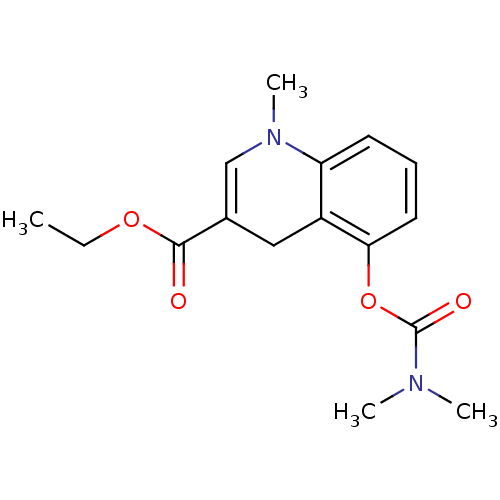
(CHEMBL1243360 | ethyl 5-(dimethylcarbamoyloxy)-1-m...)Show SMILES CCOC(=O)C1=CN(C)c2cccc(OC(=O)N(C)C)c2C1 |t:5| Show InChI InChI=1S/C16H20N2O4/c1-5-21-15(19)11-9-12-13(18(4)10-11)7-6-8-14(12)22-16(20)17(2)3/h6-8,10H,5,9H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326250
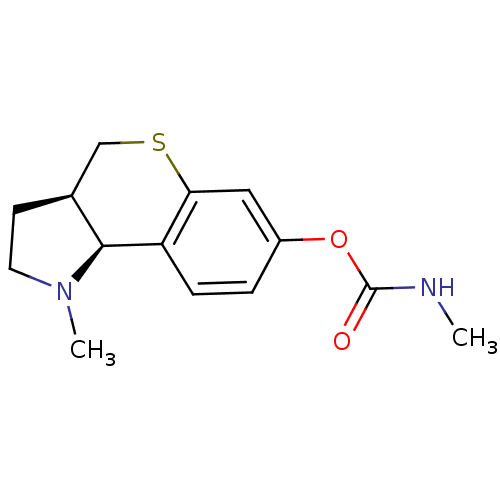
((3aR,9bS)-1-methyl-1,2,3,3a,4,9b-hexahydrothiochro...)Show SMILES CNC(=O)Oc1ccc2[C@@H]3[C@@H](CCN3C)CSc2c1 |r| Show InChI InChI=1S/C14H18N2O2S/c1-15-14(17)18-10-3-4-11-12(7-10)19-8-9-5-6-16(2)13(9)11/h3-4,7,9,13H,5-6,8H2,1-2H3,(H,15,17)/t9-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10961

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3CC)ccc1N2C |r| Show InChI InChI=1S/C22H27N3O2/c1-5-15-8-6-7-9-18(15)23-21(26)27-16-10-11-19-17(14-16)22(2)12-13-24(3)20(22)25(19)4/h6-11,14,20H,5,12-13H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10973

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3C(C)C)ccc1N2C |r| Show InChI InChI=1S/C23H29N3O2/c1-15(2)17-8-6-7-9-19(17)24-22(27)28-16-10-11-20-18(14-16)23(3)12-13-25(4)21(23)26(20)5/h6-11,14-15,21H,12-13H2,1-5H3,(H,24,27)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326251

((3aR,9bS)-1-methyl-2,3,3a,4,5,9b-hexahydro-1H-benz...)Show SMILES CNC(=O)Oc1ccc2[C@@H]3[C@@H](CCN3C)CCc2c1 |r| Show InChI InChI=1S/C15H20N2O2/c1-16-15(18)19-12-5-6-13-11(9-12)4-3-10-7-8-17(2)14(10)13/h5-6,9-10,14H,3-4,7-8H2,1-2H3,(H,16,18)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972
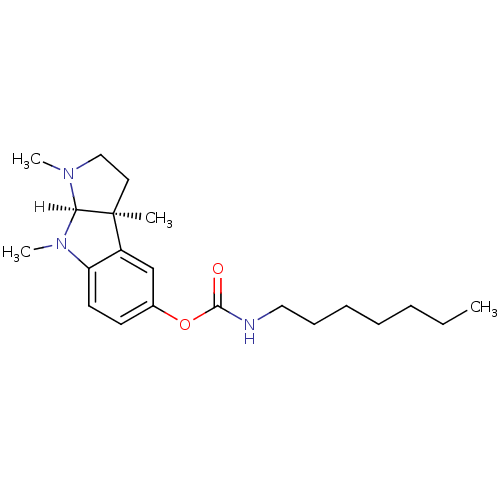
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10958
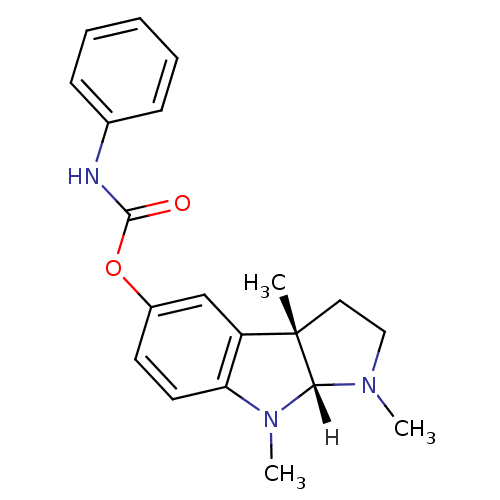
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10978

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3cc(C)ccc3C)ccc1N2C |r| Show InChI InChI=1S/C22H27N3O2/c1-14-6-7-15(2)18(12-14)23-21(26)27-16-8-9-19-17(13-16)22(3)10-11-24(4)20(22)25(19)5/h6-9,12-13,20H,10-11H2,1-5H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326252

((3aR,9bS)-1-methyl-1,2,3,3a,4,9b-hexahydrochromeno...)Show InChI InChI=1S/C14H18N2O3/c1-15-14(17)19-10-3-4-11-12(7-10)18-8-9-5-6-16(2)13(9)11/h3-4,7,9,13H,5-6,8H2,1-2H3,(H,15,17)/t9-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326253
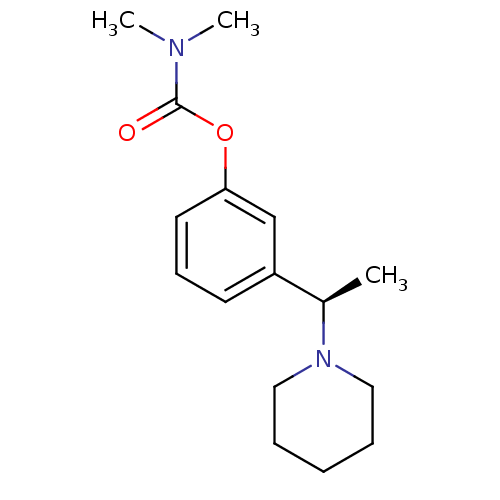
((R)-3-(1-(piperidin-1-yl)ethyl)phenyl dimethylcarb...)Show InChI InChI=1S/C16H24N2O2/c1-13(18-10-5-4-6-11-18)14-8-7-9-15(12-14)20-16(19)17(2)3/h7-9,12-13H,4-6,10-11H2,1-3H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10959

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3C)ccc1N2C |r| Show InChI InChI=1S/C21H25N3O2/c1-14-7-5-6-8-17(14)22-20(25)26-15-9-10-18-16(13-15)21(2)11-12-23(3)19(21)24(18)4/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326248

((3aS,8aS)-1,3a-dimethyl-1,2,3,3a,8,8a-hexahydropyr...)Show SMILES CN1CC[C@]2(C)[C@H]1Nc1ccc(OC(=O)Nc3ccccc3C)cc21 |r| Show InChI InChI=1S/C20H23N3O2/c1-13-6-4-5-7-16(13)22-19(24)25-14-8-9-17-15(12-14)20(2)10-11-23(3)18(20)21-17/h4-9,12,18,21H,10-11H2,1-3H3,(H,22,24)/t18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326247

((3aS,8aS)-1,3a-dimethyl-1,2,3,3a,8,8a-hexahydropyr...)Show SMILES CNC(=O)Oc1ccc2N[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C14H19N3O2/c1-14-6-7-17(3)12(14)16-11-5-4-9(8-10(11)14)19-13(18)15-2/h4-5,8,12,16H,6-7H2,1-3H3,(H,15,18)/t12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10982
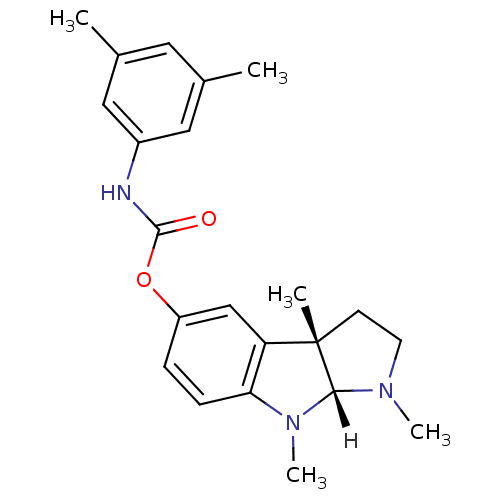
((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3cc(C)cc(C)c3)ccc1N2C |r| Show InChI InChI=1S/C22H27N3O2/c1-14-10-15(2)12-16(11-14)23-21(26)27-17-6-7-19-18(13-17)22(3)8-9-24(4)20(22)25(19)5/h6-7,10-13,20H,8-9H2,1-5H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326246

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C16H23N3O2/c1-5-17-15(20)21-11-6-7-13-12(10-11)16(2)8-9-18(3)14(16)19(13)4/h6-7,10,14H,5,8-9H2,1-4H3,(H,17,20)/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50290389

(CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...)Show InChI InChI=1S/C16H15FN4O2/c1-10-9-21(20-14-5-6-19-8-13(14)17)15-4-3-11(7-12(10)15)23-16(22)18-2/h3-9H,1-2H3,(H,18,22)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10960
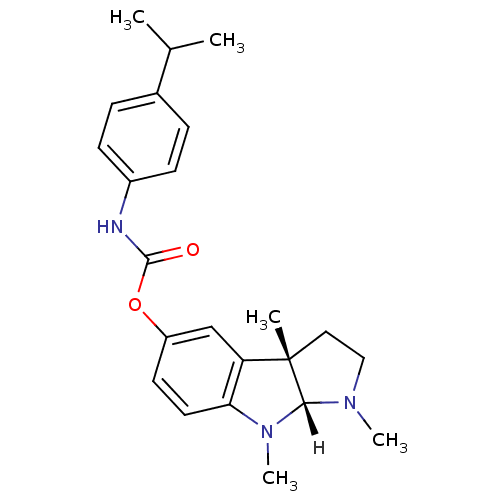
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccc(cc3)C(C)C)ccc1N2C |r| Show InChI InChI=1S/C23H29N3O2/c1-15(2)16-6-8-17(9-7-16)24-22(27)28-18-10-11-20-19(14-18)23(3)12-13-25(4)21(23)26(20)5/h6-11,14-15,21H,12-13H2,1-5H3,(H,24,27)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50292573

((2-Ethyl-phenyl)-carbamic acid (3aS,8aS)-1,3a,8-tr...)Show SMILES CCc1ccccc1NC(=O)Oc1ccc2N(C)[C@@H]3[C@@](C)(CC[N+]3(C)[O-])c2c1 |r| Show InChI InChI=1S/C22H27N3O3/c1-5-15-8-6-7-9-18(15)23-21(26)28-16-10-11-19-17(14-16)22(2)12-13-25(4,27)20(22)24(19)3/h6-11,14,20H,5,12-13H2,1-4H3,(H,23,26)/t20-,22-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326265

(CHEMBL1243298 | quilostigmine)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)N3CCc4ccccc4C3)cc21 |r| Show InChI InChI=1S/C23H27N3O2/c1-23-11-13-24(2)21(23)25(3)20-9-8-18(14-19(20)23)28-22(27)26-12-10-16-6-4-5-7-17(16)15-26/h4-9,14,21H,10-13,15H2,1-3H3/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326264

((3aS,8aR)-8-benzyl-1,3a-dimethyl-1,2,3,3a,8,8a-hex...)Show SMILES CNC(=O)Oc1ccc2N(Cc3ccccc3)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-23(3)19(21)24(14-15-7-5-4-6-8-15)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326263

((3aS,8aR)-8-benzyl-1,3a-dimethyl-1,2,3,3a,8,8a-hex...)Show SMILES CN1CC[C@]2(C)[C@H]1N(Cc1ccccc1)c1ccc(OC(=O)Nc3ccccc3)cc21 |r| Show InChI InChI=1S/C26H27N3O2/c1-26-15-16-28(2)24(26)29(18-19-9-5-3-6-10-19)23-14-13-21(17-22(23)26)31-25(30)27-20-11-7-4-8-12-20/h3-14,17,24H,15-16,18H2,1-2H3,(H,27,30)/t24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10985
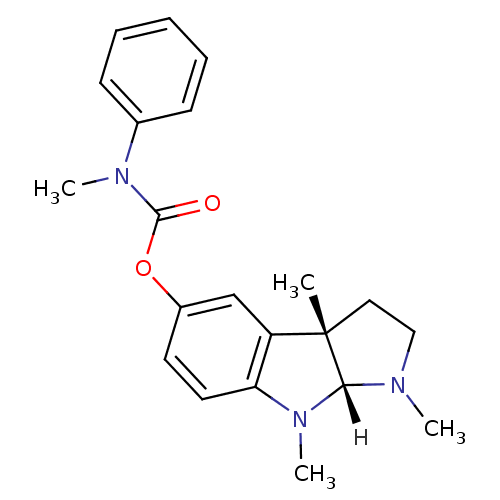
((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)c3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C21H25N3O2/c1-21-12-13-22(2)19(21)24(4)18-11-10-16(14-17(18)21)26-20(25)23(3)15-8-6-5-7-9-15/h5-11,14,19H,12-13H2,1-4H3/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326262
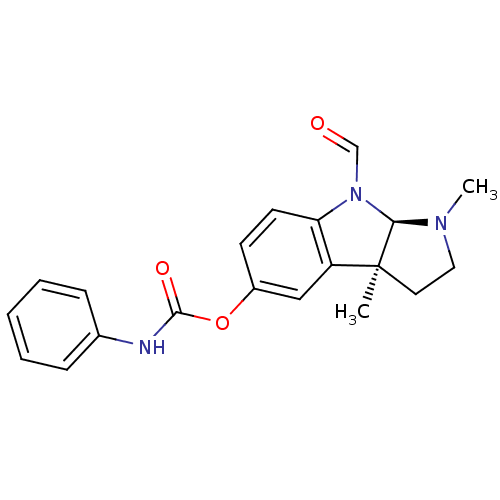
((3aS,8aR)-8-formyl-1,3a-dimethyl-1,2,3,3a,8,8a-hex...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C=O)c1ccc(OC(=O)Nc3ccccc3)cc21 |r| Show InChI InChI=1S/C20H21N3O3/c1-20-10-11-22(2)18(20)23(13-24)17-9-8-15(12-16(17)20)26-19(25)21-14-6-4-3-5-7-14/h3-9,12-13,18H,10-11H2,1-2H3,(H,21,25)/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326261

(CHEMBL1241457 | Ethyl-carbamic acid 1-methyl-8,10,...)Show InChI InChI=1S/C13H15NO5/c1-3-14-11(15)17-8-4-5-10-9(6-8)13(2)7-16-12(18-10)19-13/h4-6,12H,3,7H2,1-2H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10980

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3c(CC)cccc3CC)ccc1N2C |r| Show InChI InChI=1S/C24H31N3O2/c1-6-16-9-8-10-17(7-2)21(16)25-23(28)29-18-11-12-20-19(15-18)24(3)13-14-26(4)22(24)27(20)5/h8-12,15,22H,6-7,13-14H2,1-5H3,(H,25,28)/t22-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10988
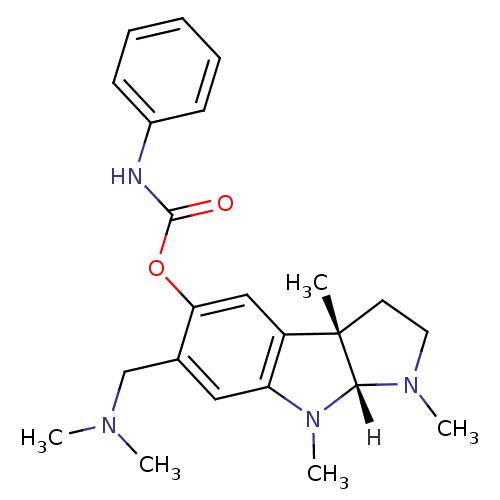
((3aS,8aR)-6-[(dimethylamino)methyl]-1,3a,8-trimeth...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)c(CN(C)C)cc1N2C |r| Show InChI InChI=1S/C23H30N4O2/c1-23-11-12-26(4)21(23)27(5)19-13-16(15-25(2)3)20(14-18(19)23)29-22(28)24-17-9-7-6-8-10-17/h6-10,13-14,21H,11-12,15H2,1-5H3,(H,24,28)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326260

((3aS,8aR)-3a-methyl-1,2,3,3a,8,8a-hexahydropyrrolo...)Show SMILES Cc1ccccc1NC(=O)Oc1ccc2N[C@H]3NCC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C19H21N3O2/c1-12-5-3-4-6-15(12)22-18(23)24-13-7-8-16-14(11-13)19(2)9-10-20-17(19)21-16/h3-8,11,17,20-21H,9-10H2,1-2H3,(H,22,23)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014112
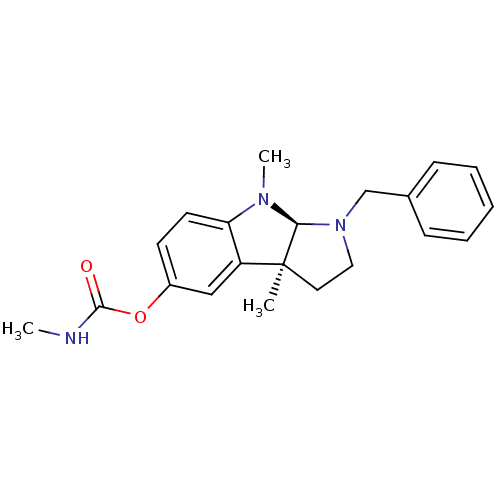
(CHEMBL74257 | Methyl-carbamic acid 1-benzyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-24(14-15-7-5-4-6-8-15)19(21)23(3)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326259

((3aS,8aR)-8-formyl-1,3a-dimethyl-1,2,3,3a,8,8a-hex...)Show SMILES CNC(=O)Oc1ccc2N(C=O)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H19N3O3/c1-15-6-7-17(3)13(15)18(9-19)12-5-4-10(8-11(12)15)21-14(20)16-2/h4-5,8-9,13H,6-7H2,1-3H3,(H,16,20)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326258

(2-Chlorophenylcarbamic Acid 1-Benzyl-1,2,3,4-tetra...)Show InChI InChI=1S/C23H21ClN2O2/c24-20-10-4-5-11-21(20)25-23(27)28-19-12-13-22-18(15-19)9-6-14-26(22)16-17-7-2-1-3-8-17/h1-5,7-8,10-13,15H,6,9,14,16H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326257
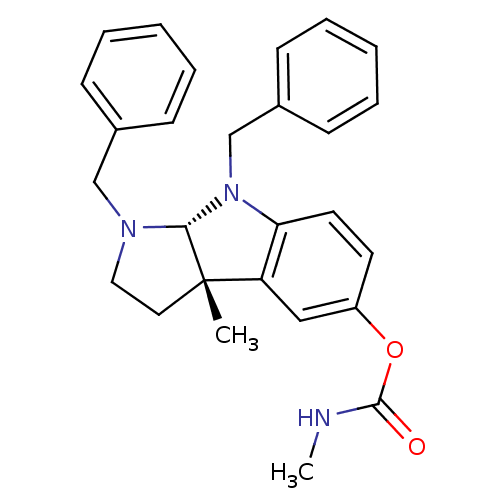
((3aS,8aR)-1,8-dibenzyl-3a-methyl-1,2,3,3a,8,8a-hex...)Show SMILES CNC(=O)Oc1ccc2N(Cc3ccccc3)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C27H29N3O2/c1-27-15-16-29(18-20-9-5-3-6-10-20)25(27)30(19-21-11-7-4-8-12-21)24-14-13-22(17-23(24)27)32-26(31)28-2/h3-14,17,25H,15-16,18-19H2,1-2H3,(H,28,31)/t25-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620
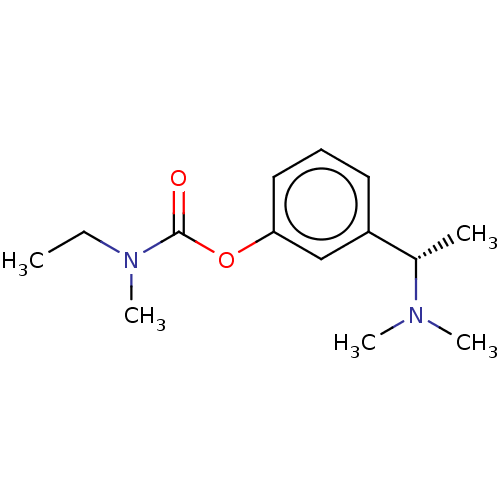
((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326256

((4-Isopropyl-phenyl)-carbamic acid 1-methyl-8,10,1...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3OC4OCC(C)(O4)c3c2)cc1 Show InChI InChI=1S/C20H21NO5/c1-12(2)13-4-6-14(7-5-13)21-18(22)24-15-8-9-17-16(10-15)20(3)11-23-19(25-17)26-20/h4-10,12,19H,11H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326255
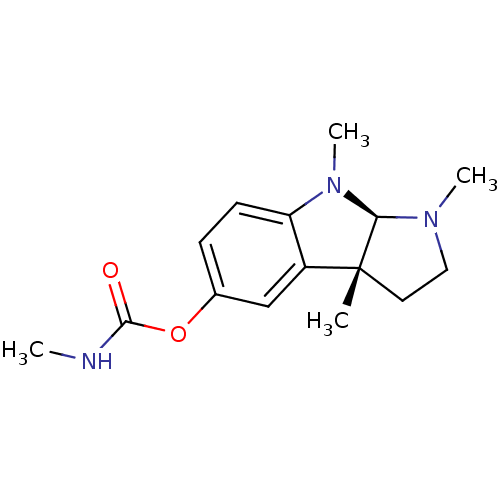
((3aR,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50326254

((3aS,8aR)-1,8-dibenzyl-3a-methyl-1,2,3,3a,8,8a-hex...)Show SMILES C[C@@]12CCN(Cc3ccccc3)[C@@H]1N(Cc1ccccc1)c1ccc(OC(=O)Nc3ccccc3)cc21 |r| Show InChI InChI=1S/C32H31N3O2/c1-32-19-20-34(22-24-11-5-2-6-12-24)30(32)35(23-25-13-7-3-8-14-25)29-18-17-27(21-28(29)32)37-31(36)33-26-15-9-4-10-16-26/h2-18,21,30H,19-20,22-23H2,1H3,(H,33,36)/t30-,32+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellmans test |
J Med Chem 53: 6490-505 (2010)
Article DOI: 10.1021/jm100573q
BindingDB Entry DOI: 10.7270/Q23R0T3D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data