 Found 106 hits Enz. Inhib. hit(s) with all data for entry = 50032349
Found 106 hits Enz. Inhib. hit(s) with all data for entry = 50032349 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50269055
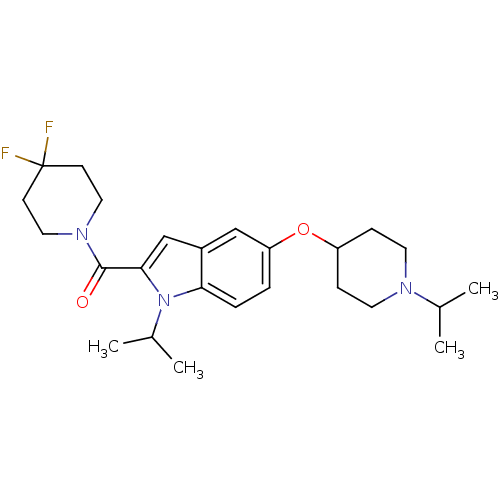
((4,4-Difluoropiperidin-1-yl)[1-isopropyl-5-(1-isop...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n(C(C)C)c(cc2c1)C(=O)N1CCC(F)(F)CC1 Show InChI InChI=1S/C25H35F2N3O2/c1-17(2)28-11-7-20(8-12-28)32-21-5-6-22-19(15-21)16-23(30(22)18(3)4)24(31)29-13-9-25(26,27)10-14-29/h5-6,15-18,20H,7-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327479
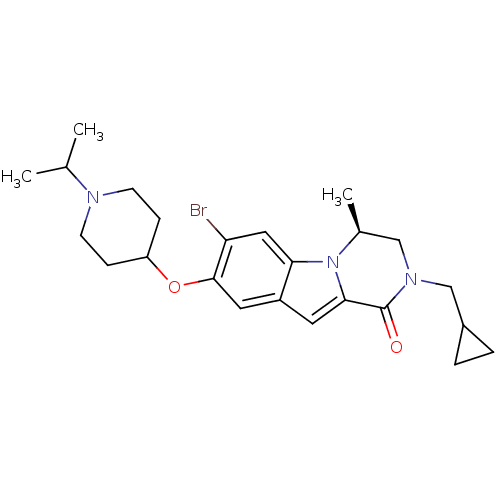
((S)-7-bromo-2-(cyclopropylmethyl)-8-(1-isopropylpi...)Show SMILES CC(C)N1CCC(CC1)Oc1cc2cc3C(=O)N(CC4CC4)C[C@H](C)n3c2cc1Br |r| Show InChI InChI=1S/C24H32BrN3O2/c1-15(2)26-8-6-19(7-9-26)30-23-11-18-10-22-24(29)27(14-17-4-5-17)13-16(3)28(22)21(18)12-20(23)25/h10-12,15-17,19H,4-9,13-14H2,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327478
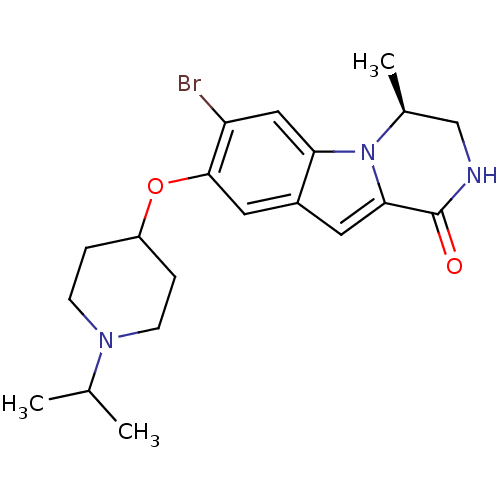
((S)-7-bromo-8-(1-isopropylpiperidin-4-yloxy)-4-met...)Show SMILES CC(C)N1CCC(CC1)Oc1cc2cc3C(=O)NC[C@H](C)n3c2cc1Br |r| Show InChI InChI=1S/C20H26BrN3O2/c1-12(2)23-6-4-15(5-7-23)26-19-9-14-8-18-20(25)22-11-13(3)24(18)17(14)10-16(19)21/h8-10,12-13,15H,4-7,11H2,1-3H3,(H,22,25)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327477
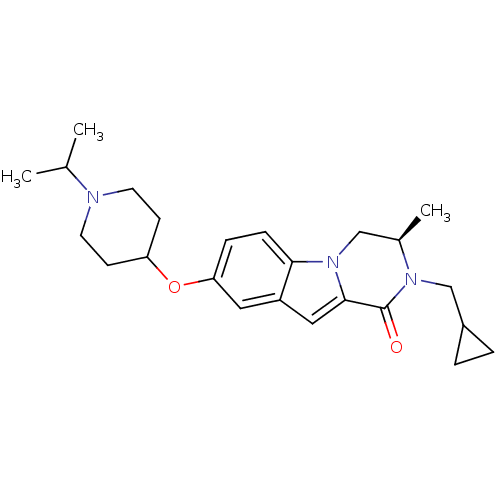
((R)-2-(cyclopropylmethyl)-8-(1-isopropylpiperidin-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3C[C@@H](C)N(CC4CC4)C(=O)c3cc2c1 |r| Show InChI InChI=1S/C24H33N3O2/c1-16(2)25-10-8-20(9-11-25)29-21-6-7-22-19(12-21)13-23-24(28)26(15-18-4-5-18)17(3)14-27(22)23/h6-7,12-13,16-18,20H,4-5,8-11,14-15H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327473
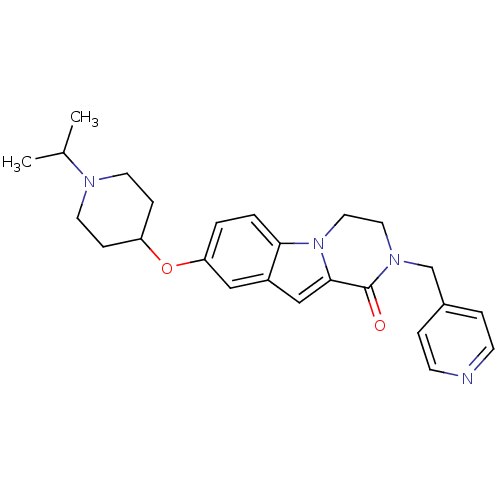
(8-(1-isopropylpiperidin-4-yloxy)-2-(pyridin-4-ylme...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccncc4)C(=O)c3cc2c1 Show InChI InChI=1S/C25H30N4O2/c1-18(2)27-11-7-21(8-12-27)31-22-3-4-23-20(15-22)16-24-25(30)28(13-14-29(23)24)17-19-5-9-26-10-6-19/h3-6,9-10,15-16,18,21H,7-8,11-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327486
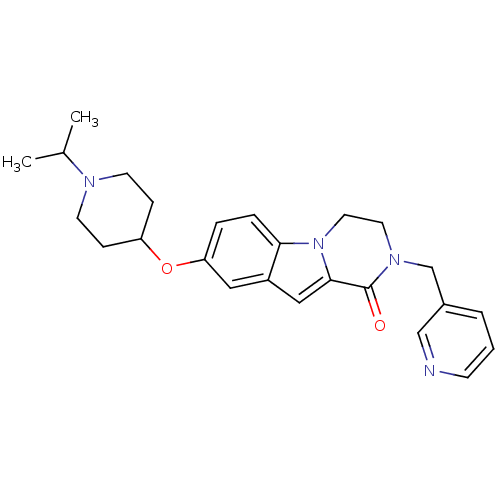
(8-(1-isopropylpiperidin-4-yloxy)-2-(pyridin-3-ylme...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4cccnc4)C(=O)c3cc2c1 Show InChI InChI=1S/C25H30N4O2/c1-18(2)27-10-7-21(8-11-27)31-22-5-6-23-20(14-22)15-24-25(30)28(12-13-29(23)24)17-19-4-3-9-26-16-19/h3-6,9,14-16,18,21H,7-8,10-13,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327487
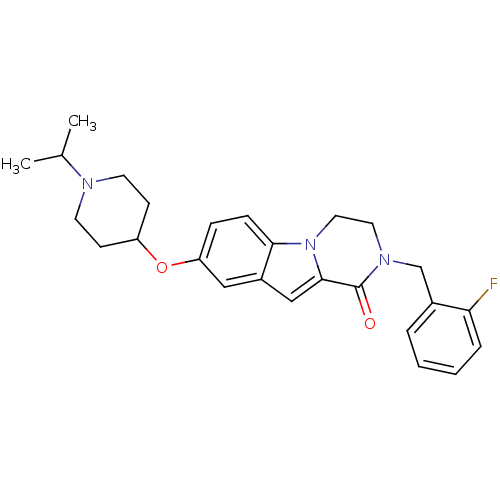
(2-(2-fluorobenzyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccccc4F)C(=O)c3cc2c1 Show InChI InChI=1S/C26H30FN3O2/c1-18(2)28-11-9-21(10-12-28)32-22-7-8-24-20(15-22)16-25-26(31)29(13-14-30(24)25)17-19-5-3-4-6-23(19)27/h3-8,15-16,18,21H,9-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327488
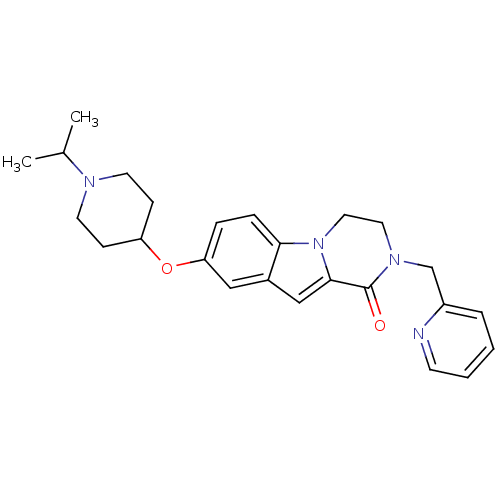
(8-(1-isopropylpiperidin-4-yloxy)-2-(pyridin-2-ylme...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccccn4)C(=O)c3cc2c1 Show InChI InChI=1S/C25H30N4O2/c1-18(2)27-11-8-21(9-12-27)31-22-6-7-23-19(15-22)16-24-25(30)28(13-14-29(23)24)17-20-5-3-4-10-26-20/h3-7,10,15-16,18,21H,8-9,11-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327475

((S)-8-(1-isopropylpiperidin-4-yloxy)-3-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3C[C@H](C)NC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-14(3)12-23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327480
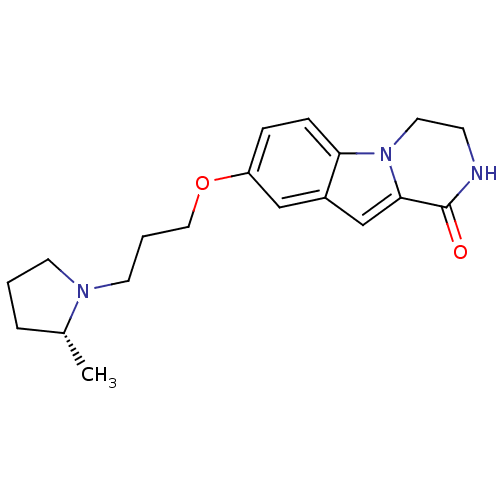
((R)-8-(3-(2-methylpyrrolidin-1-yl)propoxy)-3,4-dih...)Show SMILES C[C@@H]1CCCN1CCCOc1ccc2n3CCNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C19H25N3O2/c1-14-4-2-8-21(14)9-3-11-24-16-5-6-17-15(12-16)13-18-19(23)20-7-10-22(17)18/h5-6,12-14H,2-4,7-11H2,1H3,(H,20,23)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327483
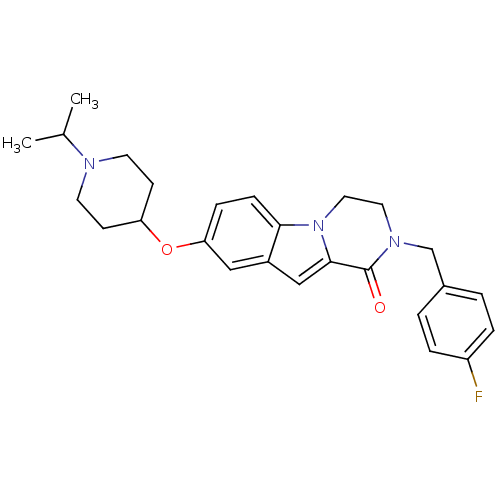
(2-(4-fluorobenzyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccc(F)cc4)C(=O)c3cc2c1 Show InChI InChI=1S/C26H30FN3O2/c1-18(2)28-11-9-22(10-12-28)32-23-7-8-24-20(15-23)16-25-26(31)29(13-14-30(24)25)17-19-3-5-21(27)6-4-19/h3-8,15-16,18,22H,9-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327472

(2-(cyclopropylmethyl)-8-(1-isopropylpiperidin-4-yl...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(CC4CC4)C(=O)c3cc2c1 Show InChI InChI=1S/C23H31N3O2/c1-16(2)24-9-7-19(8-10-24)28-20-5-6-21-18(13-20)14-22-23(27)25(11-12-26(21)22)15-17-3-4-17/h5-6,13-14,16-17,19H,3-4,7-12,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327474
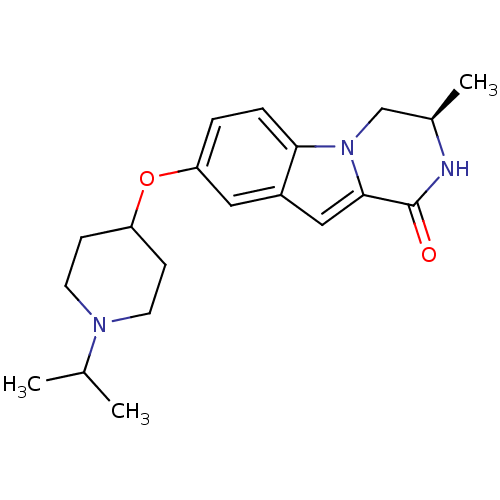
((R)-8-(1-isopropylpiperidin-4-yloxy)-3-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3C[C@@H](C)NC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-14(3)12-23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327489
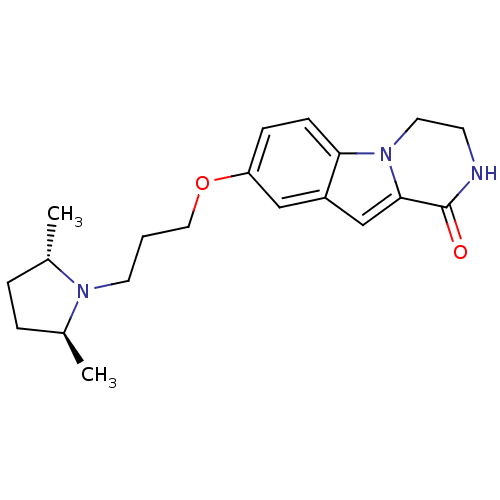
(8-(3-((2S,5S)-2,5-dimethylpyrrolidin-1-yl)propoxy)...)Show SMILES C[C@H]1CC[C@H](C)N1CCCOc1ccc2n3CCNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-14-4-5-15(2)22(14)9-3-11-25-17-6-7-18-16(12-17)13-19-20(24)21-8-10-23(18)19/h6-7,12-15H,3-5,8-11H2,1-2H3,(H,21,24)/t14-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50327477

((R)-2-(cyclopropylmethyl)-8-(1-isopropylpiperidin-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3C[C@@H](C)N(CC4CC4)C(=O)c3cc2c1 |r| Show InChI InChI=1S/C24H33N3O2/c1-16(2)25-10-8-20(9-11-25)29-21-6-7-22-19(12-21)13-23-24(28)26(15-18-4-5-18)17(3)14-27(22)23/h6-7,12-13,16-18,20H,4-5,8-11,14-15H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327490
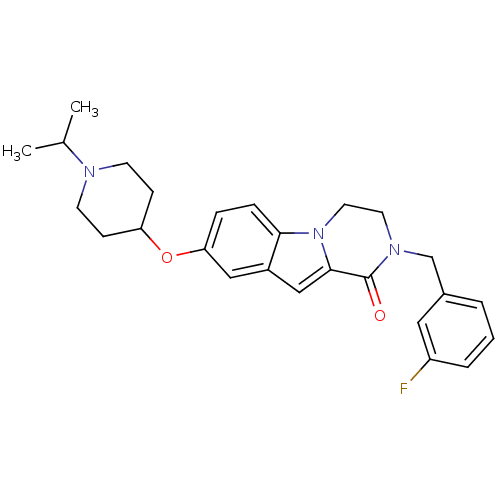
(2-(3-fluorobenzyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4cccc(F)c4)C(=O)c3cc2c1 Show InChI InChI=1S/C26H30FN3O2/c1-18(2)28-10-8-22(9-11-28)32-23-6-7-24-20(15-23)16-25-26(31)29(12-13-30(24)25)17-19-4-3-5-21(27)14-19/h3-7,14-16,18,22H,8-13,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327471
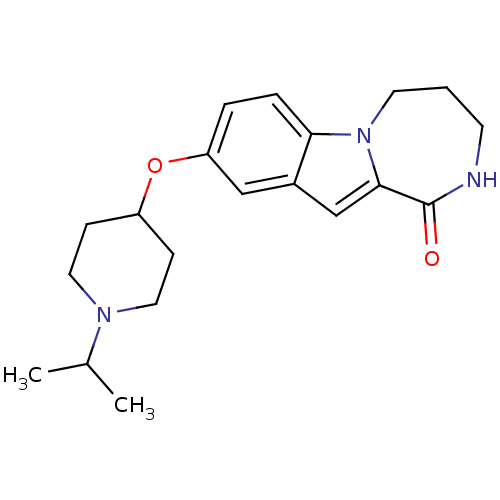
(9-(1-isopropylpiperidin-4-yloxy)-2,3,4,5-tetrahydr...)Show InChI InChI=1S/C20H27N3O2/c1-14(2)22-10-6-16(7-11-22)25-17-4-5-18-15(12-17)13-19-20(24)21-8-3-9-23(18)19/h4-5,12-14,16H,3,6-11H2,1-2H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327491
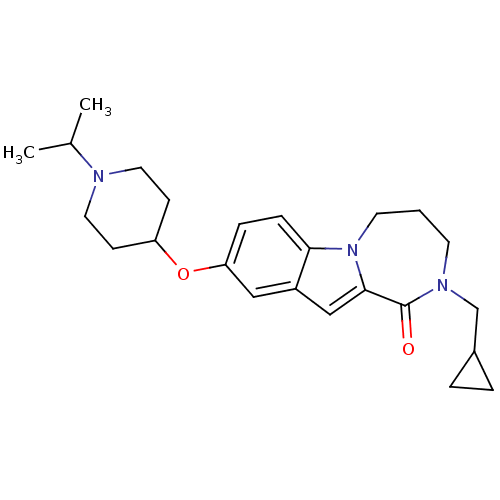
(2-(cyclopropylmethyl)-9-(1-isopropylpiperidin-4-yl...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCCN(CC4CC4)C(=O)c3cc2c1 Show InChI InChI=1S/C24H33N3O2/c1-17(2)25-12-8-20(9-13-25)29-21-6-7-22-19(14-21)15-23-24(28)26(16-18-4-5-18)10-3-11-27(22)23/h6-7,14-15,17-18,20H,3-5,8-13,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327492
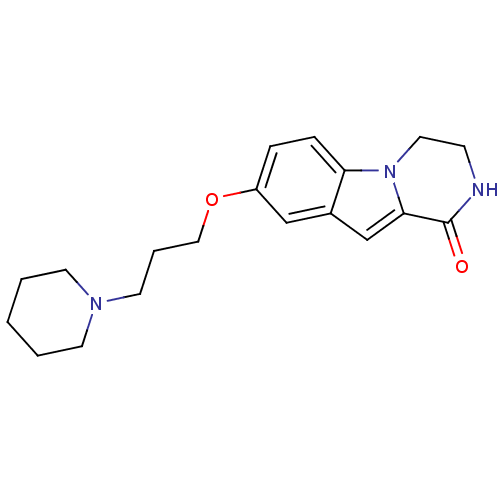
(8-(3-(piperidin-1-yl)propoxy)-3,4-dihydropyrazino[...)Show InChI InChI=1S/C19H25N3O2/c23-19-18-14-15-13-16(5-6-17(15)22(18)11-7-20-19)24-12-4-10-21-8-2-1-3-9-21/h5-6,13-14H,1-4,7-12H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327493
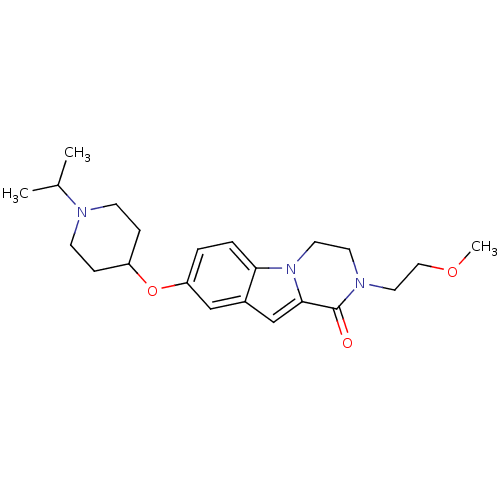
(8-(1-isopropylpiperidin-4-yloxy)-2-(2-methoxyethyl...)Show SMILES COCCN1CCn2c(cc3cc(OC4CCN(CC4)C(C)C)ccc23)C1=O Show InChI InChI=1S/C22H31N3O3/c1-16(2)23-8-6-18(7-9-23)28-19-4-5-20-17(14-19)15-21-22(26)24(12-13-27-3)10-11-25(20)21/h4-5,14-16,18H,6-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327494
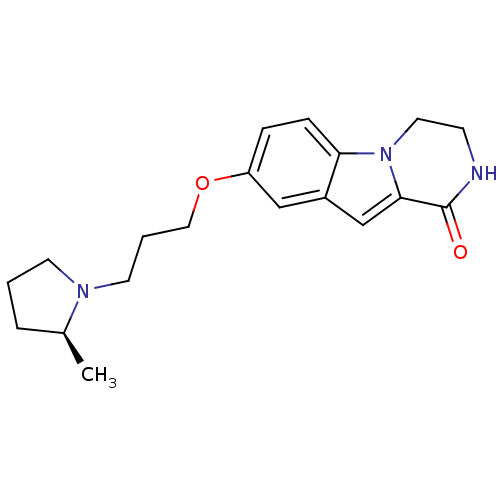
((S)-8-(3-(2-methylpyrrolidin-1-yl)propoxy)-3,4-dih...)Show SMILES C[C@H]1CCCN1CCCOc1ccc2n3CCNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C19H25N3O2/c1-14-4-2-8-21(14)9-3-11-24-16-5-6-17-15(12-16)13-18-19(23)20-7-10-22(17)18/h5-6,12-14H,2-4,7-11H2,1H3,(H,20,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327481
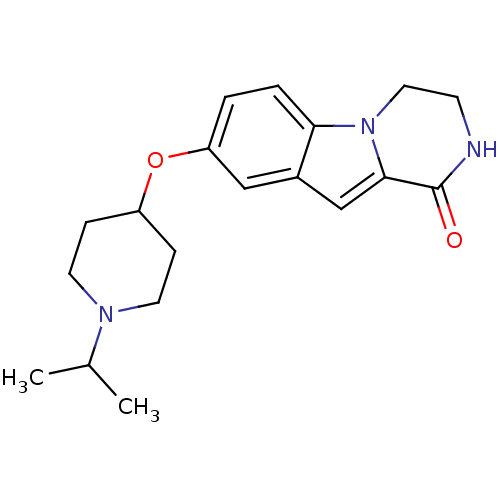
(8-(1-isopropylpiperidin-4-yloxy)-3,4-dihydropyrazi...)Show InChI InChI=1S/C19H25N3O2/c1-13(2)21-8-5-15(6-9-21)24-16-3-4-17-14(11-16)12-18-19(23)20-7-10-22(17)18/h3-4,11-13,15H,5-10H2,1-2H3,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327485
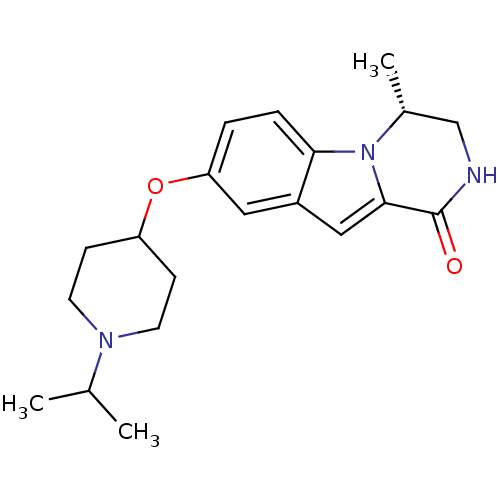
((R)-8-(1-isopropylpiperidin-4-yloxy)-4-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3[C@H](C)CNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-12-14(3)23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327495

(2-isopropyl-8-(1-isopropylpiperidin-4-yloxy)-3,4-d...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(C(C)C)C(=O)c3cc2c1 Show InChI InChI=1S/C22H31N3O2/c1-15(2)23-9-7-18(8-10-23)27-19-5-6-20-17(13-19)14-21-22(26)24(16(3)4)11-12-25(20)21/h5-6,13-16,18H,7-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327482
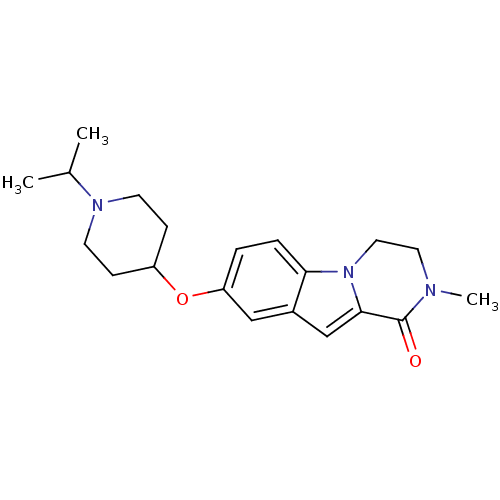
(8-(1-isopropylpiperidin-4-yloxy)-2-methyl-3,4-dihy...)Show InChI InChI=1S/C20H27N3O2/c1-14(2)22-8-6-16(7-9-22)25-17-4-5-18-15(12-17)13-19-20(24)21(3)10-11-23(18)19/h4-5,12-14,16H,6-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327496
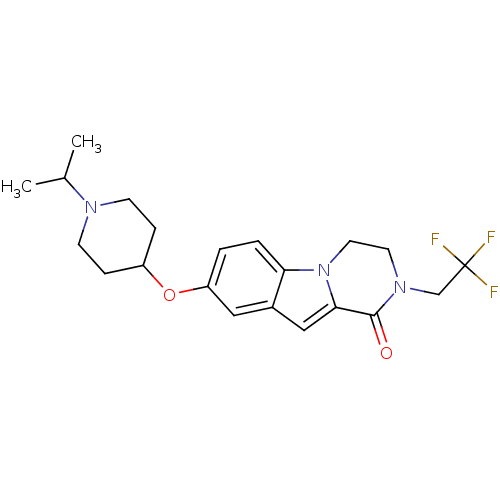
(8-(1-isopropylpiperidin-4-yloxy)-2-(2,2,2-trifluor...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(CC(F)(F)F)C(=O)c3cc2c1 Show InChI InChI=1S/C21H26F3N3O2/c1-14(2)25-7-5-16(6-8-25)29-17-3-4-18-15(11-17)12-19-20(28)26(9-10-27(18)19)13-21(22,23)24/h3-4,11-12,14,16H,5-10,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327476
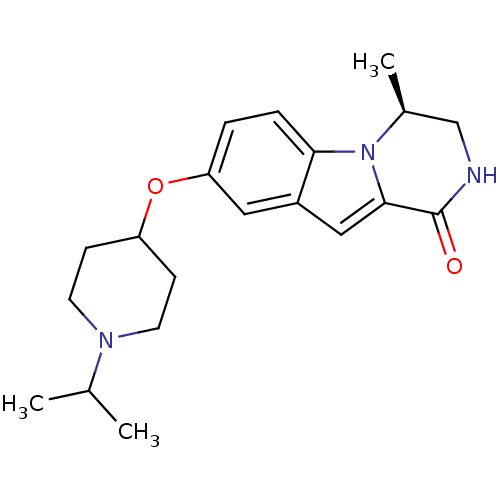
((S)-8-(1-isopropylpiperidin-4-yloxy)-4-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3[C@@H](C)CNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-12-14(3)23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327484
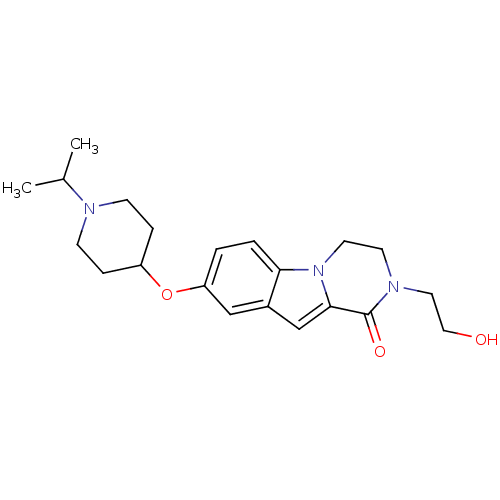
(2-(2-hydroxyethyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(CCO)C(=O)c3cc2c1 Show InChI InChI=1S/C21H29N3O3/c1-15(2)22-7-5-17(6-8-22)27-18-3-4-19-16(13-18)14-20-21(26)23(11-12-25)9-10-24(19)20/h3-4,13-15,17,25H,5-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327497
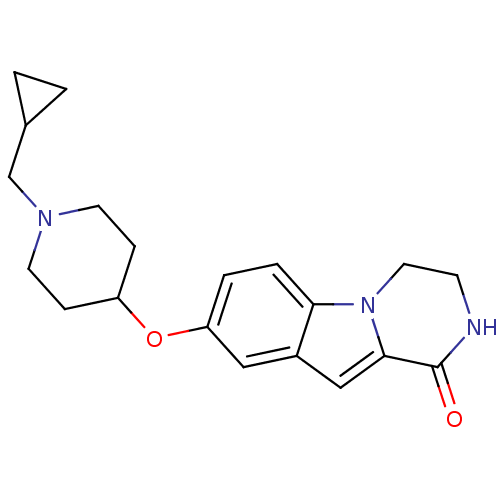
(8-(1-(cyclopropylmethyl)piperidin-4-yloxy)-3,4-dih...)Show InChI InChI=1S/C20H25N3O2/c24-20-19-12-15-11-17(3-4-18(15)23(19)10-7-21-20)25-16-5-8-22(9-6-16)13-14-1-2-14/h3-4,11-12,14,16H,1-2,5-10,13H2,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50327498
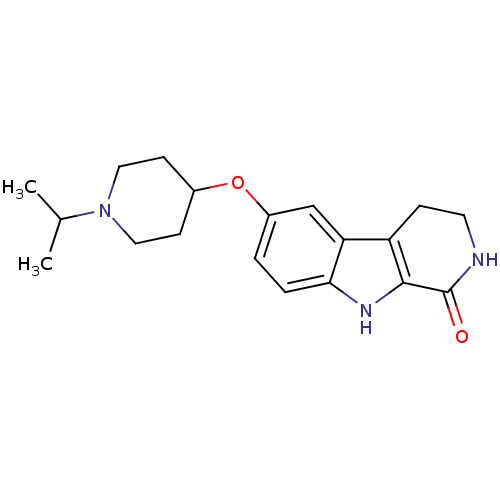
(6-(1-isopropylpiperidin-4-yloxy)-2,3,4,9-tetrahydr...)Show InChI InChI=1S/C19H25N3O2/c1-12(2)22-9-6-13(7-10-22)24-14-3-4-17-16(11-14)15-5-8-20-19(23)18(15)21-17/h3-4,11-13,21H,5-10H2,1-2H3,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50327484

(2-(2-hydroxyethyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(CCO)C(=O)c3cc2c1 Show InChI InChI=1S/C21H29N3O3/c1-15(2)22-7-5-17(6-8-22)27-18-3-4-19-16(13-18)14-20-21(26)23(11-12-25)9-10-24(19)20/h3-4,13-15,17,25H,5-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50327481

(8-(1-isopropylpiperidin-4-yloxy)-3,4-dihydropyrazi...)Show InChI InChI=1S/C19H25N3O2/c1-13(2)21-8-5-15(6-9-21)24-16-3-4-17-14(11-16)12-18-19(23)20-7-10-22(17)18/h3-4,11-13,15H,5-10H2,1-2H3,(H,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50269055

((4,4-Difluoropiperidin-1-yl)[1-isopropyl-5-(1-isop...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n(C(C)C)c(cc2c1)C(=O)N1CCC(F)(F)CC1 Show InChI InChI=1S/C25H35F2N3O2/c1-17(2)28-11-7-20(8-12-28)32-21-5-6-22-19(15-21)16-23(30(22)18(3)4)24(31)29-13-9-25(26,27)10-14-29/h5-6,15-18,20H,7-14H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50327473

(8-(1-isopropylpiperidin-4-yloxy)-2-(pyridin-4-ylme...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccncc4)C(=O)c3cc2c1 Show InChI InChI=1S/C25H30N4O2/c1-18(2)27-11-7-21(8-12-27)31-22-3-4-23-20(15-22)16-24-25(30)28(13-14-29(23)24)17-19-5-9-26-10-6-19/h3-6,9-10,15-16,18,21H,7-8,11-14,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50327482

(8-(1-isopropylpiperidin-4-yloxy)-2-methyl-3,4-dihy...)Show InChI InChI=1S/C20H27N3O2/c1-14(2)22-8-6-16(7-9-22)25-17-4-5-18-15(12-17)13-19-20(24)21(3)10-11-23(18)19/h4-5,12-14,16H,6-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50327485

((R)-8-(1-isopropylpiperidin-4-yloxy)-4-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3[C@H](C)CNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-12-14(3)23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50327476

((S)-8-(1-isopropylpiperidin-4-yloxy)-4-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3[C@@H](C)CNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-12-14(3)23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50327483

(2-(4-fluorobenzyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccc(F)cc4)C(=O)c3cc2c1 Show InChI InChI=1S/C26H30FN3O2/c1-18(2)28-11-9-22(10-12-28)32-23-7-8-24-20(15-23)16-25-26(31)29(13-14-30(24)25)17-19-3-5-21(27)6-4-19/h3-8,15-16,18,22H,9-14,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50327480

((R)-8-(3-(2-methylpyrrolidin-1-yl)propoxy)-3,4-dih...)Show SMILES C[C@@H]1CCCN1CCCOc1ccc2n3CCNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C19H25N3O2/c1-14-4-2-8-21(14)9-3-11-24-16-5-6-17-15(12-16)13-18-19(23)20-7-10-22(17)18/h5-6,12-14H,2-4,7-11H2,1H3,(H,20,23)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50269055

((4,4-Difluoropiperidin-1-yl)[1-isopropyl-5-(1-isop...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n(C(C)C)c(cc2c1)C(=O)N1CCC(F)(F)CC1 Show InChI InChI=1S/C25H35F2N3O2/c1-17(2)28-11-7-20(8-12-28)32-21-5-6-22-19(15-21)16-23(30(22)18(3)4)24(31)29-13-9-25(26,27)10-14-29/h5-6,15-18,20H,7-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327481

(8-(1-isopropylpiperidin-4-yloxy)-3,4-dihydropyrazi...)Show InChI InChI=1S/C19H25N3O2/c1-13(2)21-8-5-15(6-9-21)24-16-3-4-17-14(11-16)12-18-19(23)20-7-10-22(17)18/h3-4,11-13,15H,5-10H2,1-2H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327471

(9-(1-isopropylpiperidin-4-yloxy)-2,3,4,5-tetrahydr...)Show InChI InChI=1S/C20H27N3O2/c1-14(2)22-10-6-16(7-11-22)25-17-4-5-18-15(12-17)13-19-20(24)21-8-3-9-23(18)19/h4-5,12-14,16H,3,6-11H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327482

(8-(1-isopropylpiperidin-4-yloxy)-2-methyl-3,4-dihy...)Show InChI InChI=1S/C20H27N3O2/c1-14(2)22-8-6-16(7-9-22)25-17-4-5-18-15(12-17)13-19-20(24)21(3)10-11-23(18)19/h4-5,12-14,16H,6-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327472

(2-(cyclopropylmethyl)-8-(1-isopropylpiperidin-4-yl...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(CC4CC4)C(=O)c3cc2c1 Show InChI InChI=1S/C23H31N3O2/c1-16(2)24-9-7-19(8-10-24)28-20-5-6-21-18(13-20)14-22-23(27)25(11-12-26(21)22)15-17-3-4-17/h5-6,13-14,16-17,19H,3-4,7-12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327483

(2-(4-fluorobenzyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccc(F)cc4)C(=O)c3cc2c1 Show InChI InChI=1S/C26H30FN3O2/c1-18(2)28-11-9-22(10-12-28)32-23-7-8-24-20(15-23)16-25-26(31)29(13-14-30(24)25)17-19-3-5-21(27)6-4-19/h3-8,15-16,18,22H,9-14,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327473

(8-(1-isopropylpiperidin-4-yloxy)-2-(pyridin-4-ylme...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(Cc4ccncc4)C(=O)c3cc2c1 Show InChI InChI=1S/C25H30N4O2/c1-18(2)27-11-7-21(8-12-27)31-22-3-4-23-20(15-22)16-24-25(30)28(13-14-29(23)24)17-19-5-9-26-10-6-19/h3-6,9-10,15-16,18,21H,7-8,11-14,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327484

(2-(2-hydroxyethyl)-8-(1-isopropylpiperidin-4-yloxy...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3CCN(CCO)C(=O)c3cc2c1 Show InChI InChI=1S/C21H29N3O3/c1-15(2)22-7-5-17(6-8-22)27-18-3-4-19-16(13-18)14-20-21(26)23(11-12-25)9-10-24(19)20/h3-4,13-15,17,25H,5-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327474

((R)-8-(1-isopropylpiperidin-4-yloxy)-3-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3C[C@@H](C)NC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-14(3)12-23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327475

((S)-8-(1-isopropylpiperidin-4-yloxy)-3-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3C[C@H](C)NC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-14(3)12-23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50327485

((R)-8-(1-isopropylpiperidin-4-yloxy)-4-methyl-3,4-...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc2n3[C@H](C)CNC(=O)c3cc2c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)22-8-6-16(7-9-22)25-17-4-5-18-15(10-17)11-19-20(24)21-12-14(3)23(18)19/h4-5,10-11,13-14,16H,6-9,12H2,1-3H3,(H,21,24)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5713-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.009
BindingDB Entry DOI: 10.7270/Q2571C7N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data