 Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50032486
Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50032486 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330011
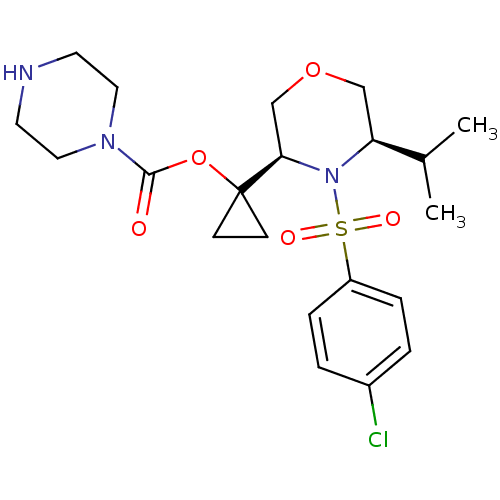
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCNCC1 |r| Show InChI InChI=1S/C21H30ClN3O5S/c1-15(2)18-13-29-14-19(25(18)31(27,28)17-5-3-16(22)4-6-17)21(7-8-21)30-20(26)24-11-9-23-10-12-24/h3-6,15,18-19,23H,7-14H2,1-2H3/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330006
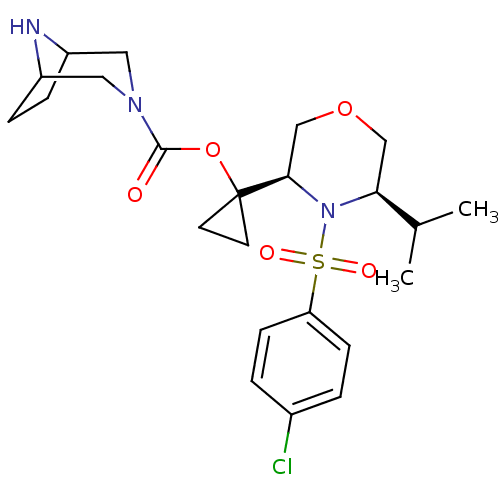
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CC2CCC(C1)N2 |r,TLB:23:25:32:28.29| Show InChI InChI=1S/C23H32ClN3O5S/c1-15(2)20-13-31-14-21(27(20)33(29,30)19-7-3-16(24)4-8-19)23(9-10-23)32-22(28)26-11-17-5-6-18(12-26)25-17/h3-4,7-8,15,17-18,20-21,25H,5-6,9-14H2,1-2H3/t17?,18?,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330016
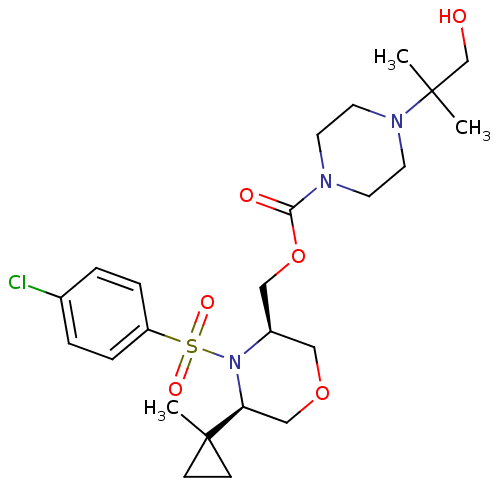
(((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...)Show SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC[C@H]1COC[C@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(C)CC1 |r| Show InChI InChI=1S/C24H36ClN3O6S/c1-23(2,17-29)27-12-10-26(11-13-27)22(30)34-15-19-14-33-16-21(24(3)8-9-24)28(19)35(31,32)20-6-4-18(25)5-7-20/h4-7,19,21,29H,8-17H2,1-3H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330017
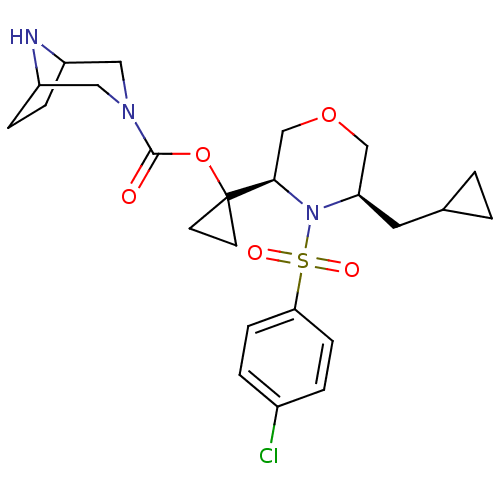
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1[C@H](CC2CC2)COC[C@@H]1C1(CC1)OC(=O)N1CC2CCC(C1)N2 |r,TLB:24:26:33:29.30| Show InChI InChI=1S/C24H32ClN3O5S/c25-17-3-7-21(8-4-17)34(30,31)28-20(11-16-1-2-16)14-32-15-22(28)24(9-10-24)33-23(29)27-12-18-5-6-19(13-27)26-18/h3-4,7-8,16,18-20,22,26H,1-2,5-6,9-15H2/t18?,19?,20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330023
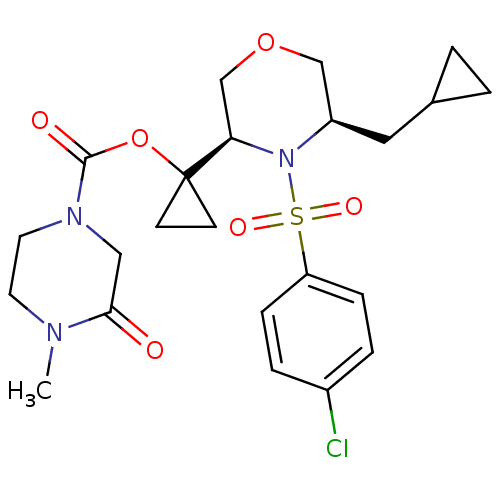
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CN1CCN(CC1=O)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H30ClN3O6S/c1-25-10-11-26(13-21(25)28)22(29)33-23(8-9-23)20-15-32-14-18(12-16-2-3-16)27(20)34(30,31)19-6-4-17(24)5-7-19/h4-7,16,18,20H,2-3,8-15H2,1H3/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330015
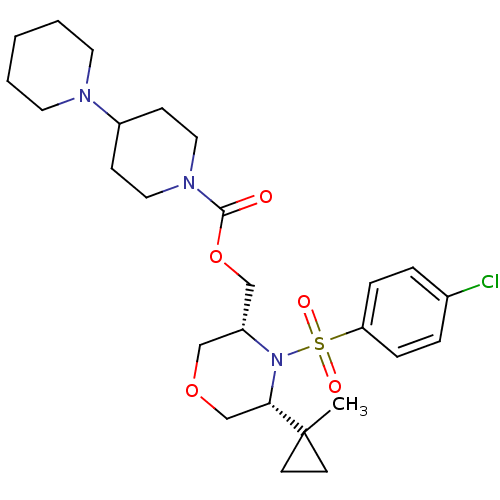
(((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...)Show SMILES CC1(CC1)[C@@H]1COC[C@H](COC(=O)N2CCC(CC2)N2CCCCC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H38ClN3O5S/c1-26(11-12-26)24-19-34-17-22(30(24)36(32,33)23-7-5-20(27)6-8-23)18-35-25(31)29-15-9-21(10-16-29)28-13-3-2-4-14-28/h5-8,21-22,24H,2-4,9-19H2,1H3/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330022
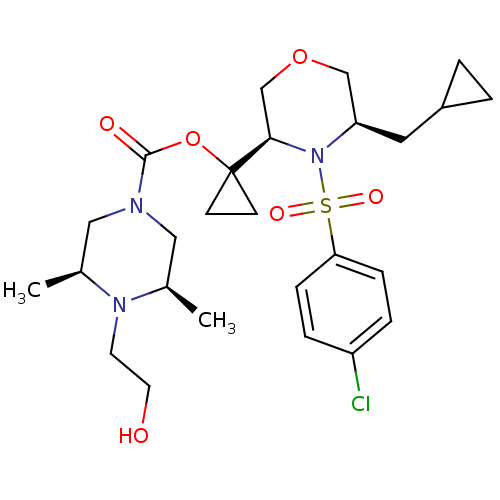
((3S,5R)-1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(c...)Show SMILES C[C@H]1CN(C[C@@H](C)N1CCO)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H38ClN3O6S/c1-18-14-28(15-19(2)29(18)11-12-31)25(32)36-26(9-10-26)24-17-35-16-22(13-20-3-4-20)30(24)37(33,34)23-7-5-21(27)6-8-23/h5-8,18-20,22,24,31H,3-4,9-17H2,1-2H3/t18-,19+,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330018
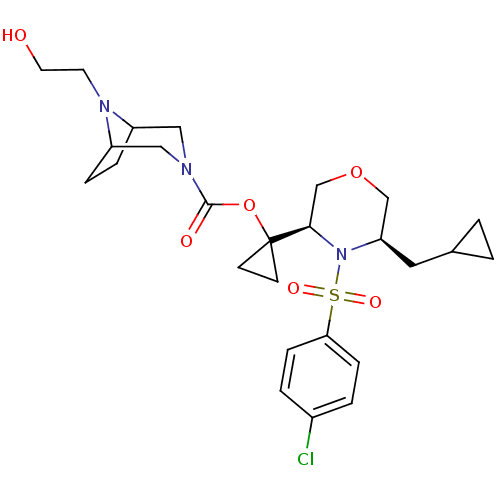
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES OCCN1C2CCC1CN(C2)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r,THB:11:9:3:5.6| Show InChI InChI=1S/C26H36ClN3O6S/c27-19-3-7-23(8-4-19)37(33,34)30-22(13-18-1-2-18)16-35-17-24(30)26(9-10-26)36-25(32)28-14-20-5-6-21(15-28)29(20)11-12-31/h3-4,7-8,18,20-22,24,31H,1-2,5-6,9-17H2/t20?,21?,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330021
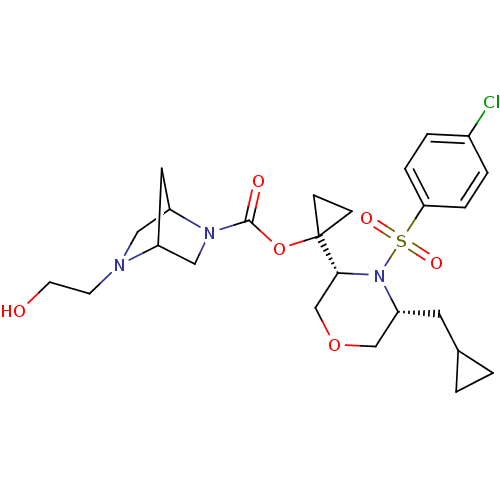
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES OCCN1CC2CC1CN2C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r,TLB:2:3:9.8:6| Show InChI InChI=1S/C25H34ClN3O6S/c26-18-3-5-22(6-4-18)36(32,33)29-21(11-17-1-2-17)15-34-16-23(29)25(7-8-25)35-24(31)28-14-19-12-20(28)13-27(19)9-10-30/h3-6,17,19-21,23,30H,1-2,7-16H2/t19?,20?,21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330014
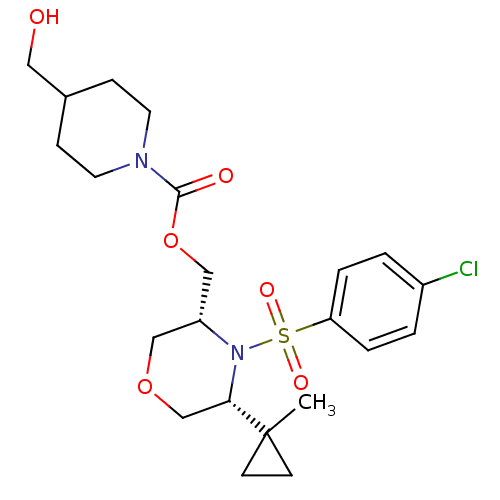
(((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...)Show SMILES CC1(CC1)[C@@H]1COC[C@H](COC(=O)N2CCC(CO)CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H31ClN2O6S/c1-22(8-9-22)20-15-30-13-18(14-31-21(27)24-10-6-16(12-26)7-11-24)25(20)32(28,29)19-4-2-17(23)3-5-19/h2-5,16,18,20,26H,6-15H2,1H3/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330010
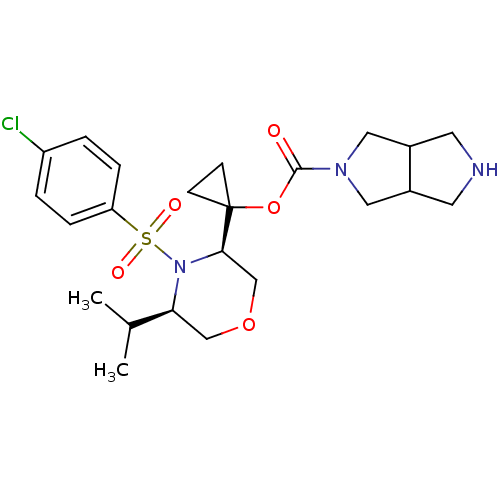
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CC2CNCC2C1 |r| Show InChI InChI=1S/C23H32ClN3O5S/c1-15(2)20-13-31-14-21(27(20)33(29,30)19-5-3-18(24)4-6-19)23(7-8-23)32-22(28)26-11-16-9-25-10-17(16)12-26/h3-6,15-17,20-21,25H,7-14H2,1-2H3/t16?,17?,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330013
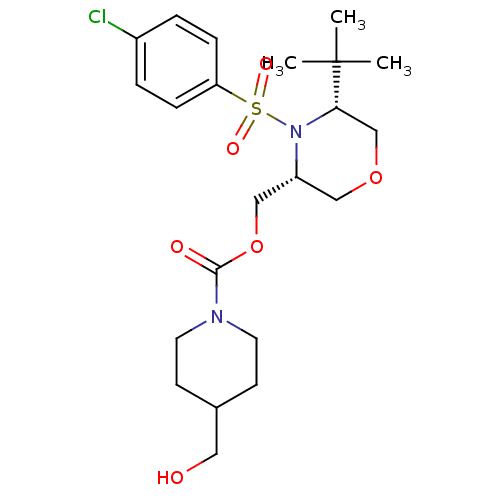
(((3R,5R)-5-tert-butyl-4-(4-chlorophenylsulfonyl)mo...)Show SMILES CC(C)(C)[C@@H]1COC[C@H](COC(=O)N2CCC(CO)CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H33ClN2O6S/c1-22(2,3)20-15-30-13-18(14-31-21(27)24-10-8-16(12-26)9-11-24)25(20)32(28,29)19-6-4-17(23)5-7-19/h4-7,16,18,20,26H,8-15H2,1-3H3/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330026
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1[C@H](CC2CC2)COC[C@@H]1C1(CC1)OC(=O)N1CCC2(CCN2)CC1 |r| Show InChI InChI=1S/C25H34ClN3O5S/c26-19-3-5-21(6-4-19)35(31,32)29-20(15-18-1-2-18)16-33-17-22(29)25(7-8-25)34-23(30)28-13-10-24(11-14-28)9-12-27-24/h3-6,18,20,22,27H,1-2,7-17H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330007
((R)-1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopro...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CC[C@@H](O)C1 |r| Show InChI InChI=1S/C21H29ClN2O6S/c1-14(2)18-12-29-13-19(24(18)31(27,28)17-5-3-15(22)4-6-17)21(8-9-21)30-20(26)23-10-7-16(25)11-23/h3-6,14,16,18-19,25H,7-13H2,1-2H3/t16-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330008
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCC(CO)CC1 |r| Show InChI InChI=1S/C23H33ClN2O6S/c1-16(2)20-14-31-15-21(26(20)33(29,30)19-5-3-18(24)4-6-19)23(9-10-23)32-22(28)25-11-7-17(13-27)8-12-25/h3-6,16-17,20-21,27H,7-15H2,1-2H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330012
(((3R,5R)-5-tert-butyl-4-(4-chlorophenylsulfonyl)mo...)Show SMILES CC(C)(C)[C@@H]1COC[C@H](COC(=O)N2CCN(CC2)C(C)(C)CO)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H38ClN3O6S/c1-23(2,3)21-16-33-14-19(28(21)35(31,32)20-8-6-18(25)7-9-20)15-34-22(30)26-10-12-27(13-11-26)24(4,5)17-29/h6-9,19,21,29H,10-17H2,1-5H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330024
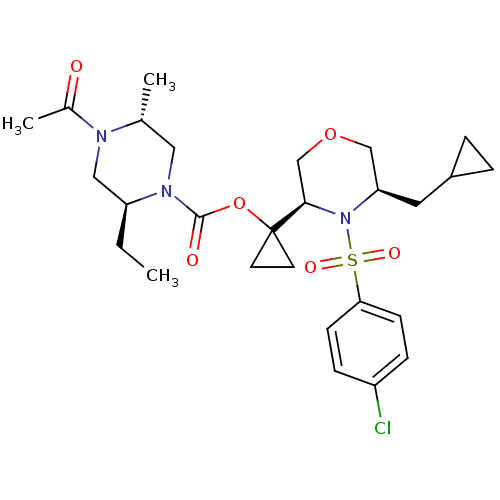
((2S,5R)-1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(c...)Show SMILES CC[C@H]1CN([C@H](C)CN1C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1)C(C)=O |r| Show InChI InChI=1S/C27H38ClN3O6S/c1-4-22-15-29(19(3)32)18(2)14-30(22)26(33)37-27(11-12-27)25-17-36-16-23(13-20-5-6-20)31(25)38(34,35)24-9-7-21(28)8-10-24/h7-10,18,20,22-23,25H,4-6,11-17H2,1-3H3/t18-,22+,23-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330019
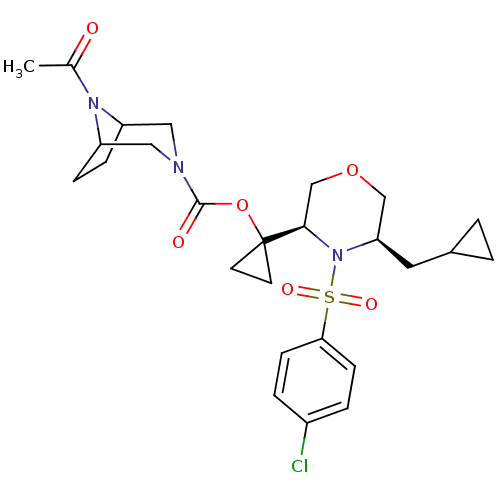
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CC(=O)N1C2CCC1CN(C2)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r,THB:11:9:3:5.6| Show InChI InChI=1S/C26H34ClN3O6S/c1-17(31)29-20-6-7-21(29)14-28(13-20)25(32)36-26(10-11-26)24-16-35-15-22(12-18-2-3-18)30(24)37(33,34)23-8-4-19(27)5-9-23/h4-5,8-9,18,20-22,24H,2-3,6-7,10-16H2,1H3/t20?,21?,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330009
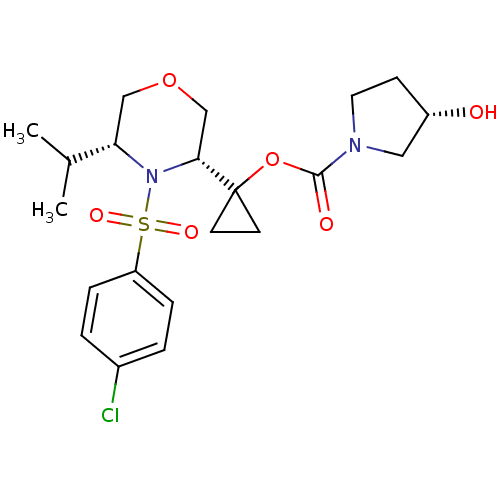
((S)-1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopro...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CC[C@H](O)C1 |r| Show InChI InChI=1S/C21H29ClN2O6S/c1-14(2)18-12-29-13-19(24(18)31(27,28)17-5-3-15(22)4-6-17)21(8-9-21)30-20(26)23-10-7-16(25)11-23/h3-6,14,16,18-19,25H,7-13H2,1-2H3/t16-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330020
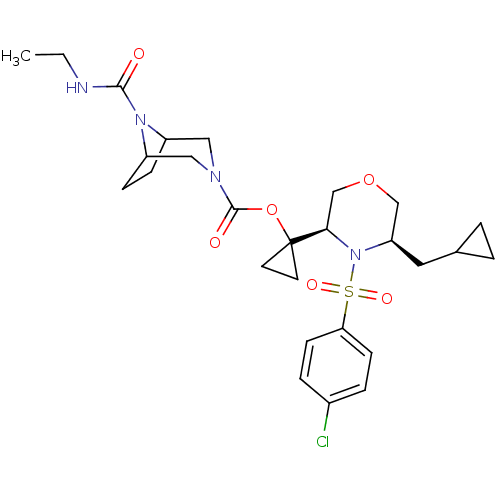
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CCNC(=O)N1C2CCC1CN(C2)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r,THB:13:11:5:7.8| Show InChI InChI=1S/C27H37ClN4O6S/c1-2-29-25(33)31-20-7-8-21(31)15-30(14-20)26(34)38-27(11-12-27)24-17-37-16-22(13-18-3-4-18)32(24)39(35,36)23-9-5-19(28)6-10-23/h5-6,9-10,18,20-22,24H,2-4,7-8,11-17H2,1H3,(H,29,33)/t20?,21?,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330025
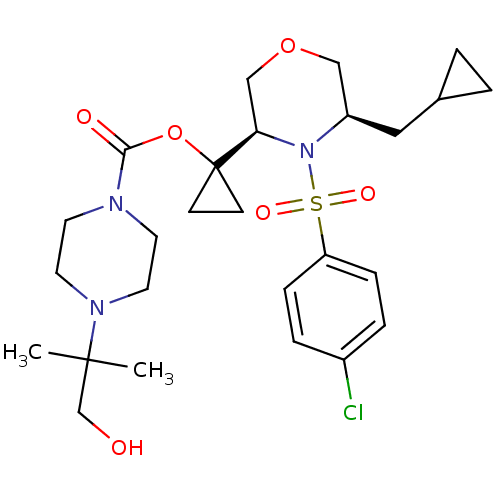
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H38ClN3O6S/c1-25(2,18-31)29-13-11-28(12-14-29)24(32)36-26(9-10-26)23-17-35-16-21(15-19-3-4-19)30(23)37(33,34)22-7-5-20(27)6-8-22/h5-8,19,21,23,31H,3-4,9-18H2,1-2H3/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330028
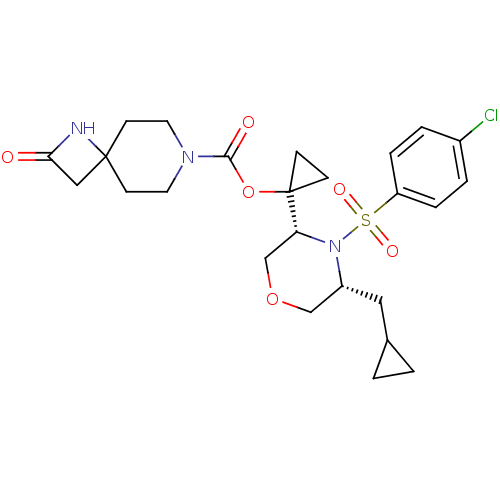
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1[C@H](CC2CC2)COC[C@@H]1C1(CC1)OC(=O)N1CCC2(CC(=O)N2)CC1 |r| Show InChI InChI=1S/C25H32ClN3O6S/c26-18-3-5-20(6-4-18)36(32,33)29-19(13-17-1-2-17)15-34-16-21(29)25(7-8-25)35-23(31)28-11-9-24(10-12-28)14-22(30)27-24/h3-6,17,19,21H,1-2,7-16H2,(H,27,30)/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330027
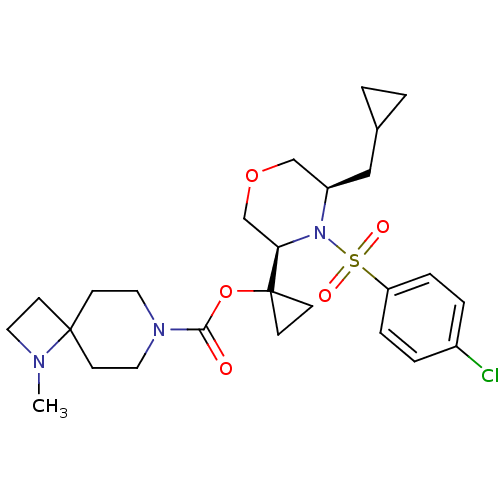
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CN1CCC11CCN(CC1)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H36ClN3O5S/c1-28-13-10-25(28)11-14-29(15-12-25)24(31)35-26(8-9-26)23-18-34-17-21(16-19-2-3-19)30(23)36(32,33)22-6-4-20(27)5-7-22/h4-7,19,21,23H,2-3,8-18H2,1H3/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50220296
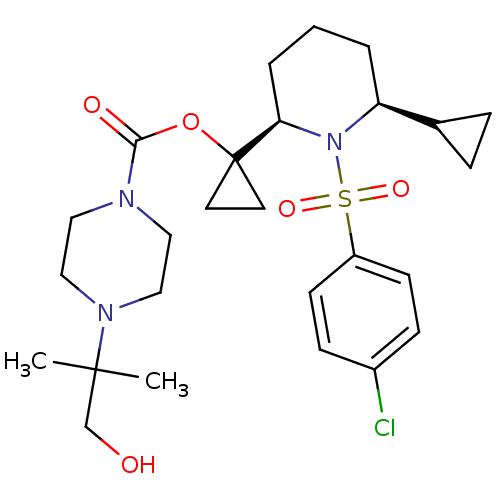
(1-((2R,6S)-1-(4-chlorophenylsulfonyl)-6-cyclopropy...)Show SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC1(CC1)[C@H]1CCC[C@@H](C2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H38ClN3O5S/c1-25(2,18-31)29-16-14-28(15-17-29)24(32)35-26(12-13-26)23-5-3-4-22(19-6-7-19)30(23)36(33,34)21-10-8-20(27)9-11-21/h8-11,19,22-23,31H,3-7,12-18H2,1-2H3/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50223252
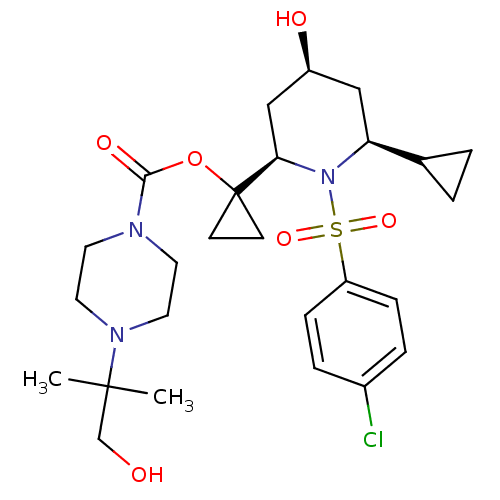
(1-((2R,4S,6S)-1-(4-chlorophenylsulfonyl)-6-cyclopr...)Show SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC1(CC1)[C@H]1C[C@@H](O)C[C@@H](C2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H38ClN3O6S/c1-25(2,17-31)29-13-11-28(12-14-29)24(33)36-26(9-10-26)23-16-20(32)15-22(18-3-4-18)30(23)37(34,35)21-7-5-19(27)6-8-21/h5-8,18,20,22-23,31-32H,3-4,9-17H2,1-2H3/t20-,22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data