 Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50032541
Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50032541 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247054
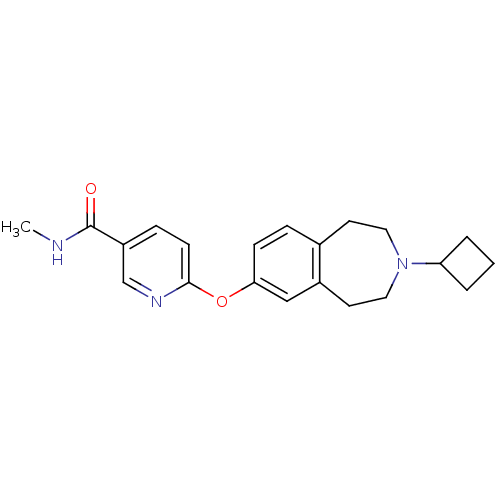
(6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)17-6-8-20(23-14-17)26-19-7-5-15-9-11-24(18-3-2-4-18)12-10-16(15)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50330789

(CHEMBL1277501 | N1-Methyl-N3-phenyl-N3-(4-(2-(pyrr...)Show InChI InChI=1S/C22H31N3O/c1-23-14-7-17-25(20-8-3-2-4-9-20)21-10-12-22(13-11-21)26-19-18-24-15-5-6-16-24/h2-4,8-13,23H,5-7,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50330789

(CHEMBL1277501 | N1-Methyl-N3-phenyl-N3-(4-(2-(pyrr...)Show InChI InChI=1S/C22H31N3O/c1-23-14-7-17-25(20-8-3-2-4-9-20)21-10-12-22(13-11-21)26-19-18-24-15-5-6-16-24/h2-4,8-13,23H,5-7,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50330791
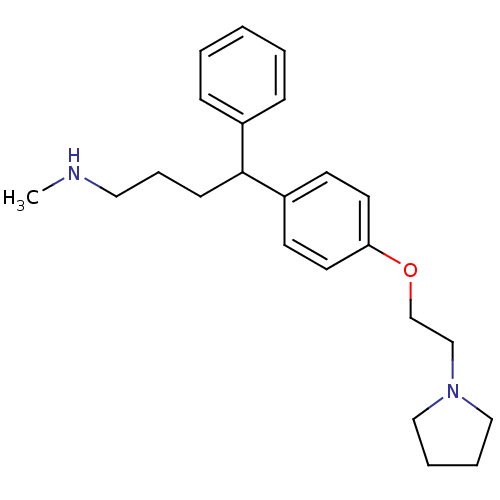
(CHEMBL1277595 | N-Methyl-4-phenyl-4-(4-(2-(pyrroli...)Show InChI InChI=1S/C23H32N2O/c1-24-15-7-10-23(20-8-3-2-4-9-20)21-11-13-22(14-12-21)26-19-18-25-16-5-6-17-25/h2-4,8-9,11-14,23-24H,5-7,10,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50330790
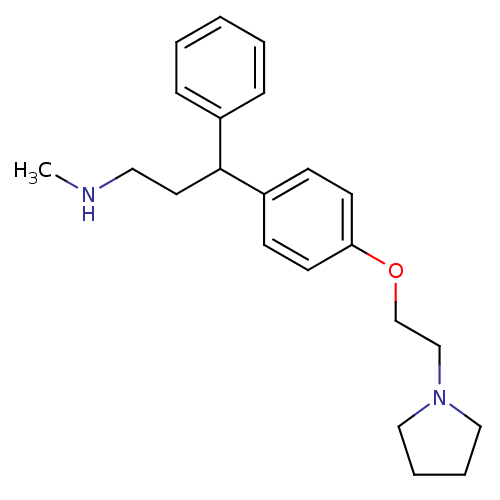
(CHEMBL1277594 | N-Methyl-3-phenyl-3-(4-(2-(pyrroli...)Show InChI InChI=1S/C22H30N2O/c1-23-14-13-22(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-24-15-5-6-16-24/h2-4,7-12,22-23H,5-6,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50330788
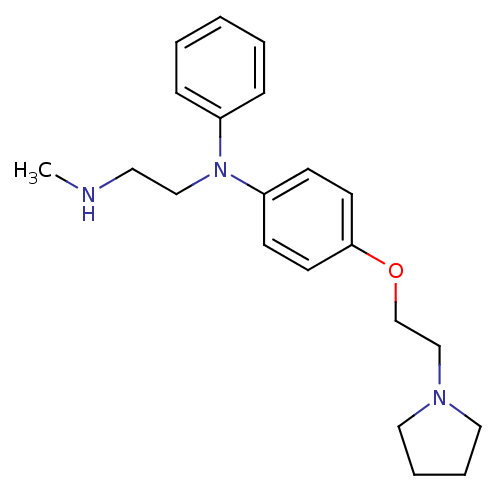
(CHEMBL1277500 | N1-Methyl-N2-phenyl-N2-(4-(2-(pyrr...)Show InChI InChI=1S/C21H29N3O/c1-22-13-16-24(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-23-14-5-6-15-23/h2-4,7-12,22H,5-6,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50330792

(CHEMBL1277686 | N-methyl-3-(naphthalen-1-yl)-3-phe...)Show InChI InChI=1S/C20H21N/c1-21-15-14-19(16-8-3-2-4-9-16)20-13-7-11-17-10-5-6-12-18(17)20/h2-13,19,21H,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50330791

(CHEMBL1277595 | N-Methyl-4-phenyl-4-(4-(2-(pyrroli...)Show InChI InChI=1S/C23H32N2O/c1-24-15-7-10-23(20-8-3-2-4-9-20)21-11-13-22(14-12-21)26-19-18-25-16-5-6-17-25/h2-4,8-9,11-14,23-24H,5-7,10,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50330790

(CHEMBL1277594 | N-Methyl-3-phenyl-3-(4-(2-(pyrroli...)Show InChI InChI=1S/C22H30N2O/c1-23-14-13-22(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-24-15-5-6-16-24/h2-4,7-12,22-23H,5-6,13-18H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50330790

(CHEMBL1277594 | N-Methyl-3-phenyl-3-(4-(2-(pyrroli...)Show InChI InChI=1S/C22H30N2O/c1-23-14-13-22(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-24-15-5-6-16-24/h2-4,7-12,22-23H,5-6,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50330788

(CHEMBL1277500 | N1-Methyl-N2-phenyl-N2-(4-(2-(pyrr...)Show InChI InChI=1S/C21H29N3O/c1-22-13-16-24(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-23-14-5-6-15-23/h2-4,7-12,22H,5-6,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50330787
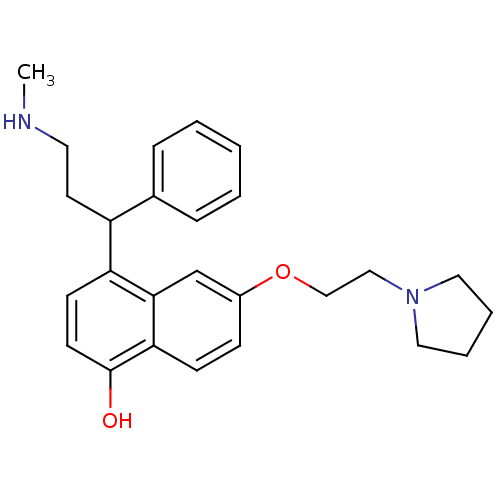
(4-(3-(Methylamino)-1-phenylpropyl)-6-(2-(pyrrolidi...)Show SMILES CNCCC(c1ccccc1)c1ccc(O)c2ccc(OCCN3CCCC3)cc12 Show InChI InChI=1S/C26H32N2O2/c1-27-14-13-22(20-7-3-2-4-8-20)23-11-12-26(29)24-10-9-21(19-25(23)24)30-18-17-28-15-5-6-16-28/h2-4,7-12,19,22,27,29H,5-6,13-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50330787

(4-(3-(Methylamino)-1-phenylpropyl)-6-(2-(pyrrolidi...)Show SMILES CNCCC(c1ccccc1)c1ccc(O)c2ccc(OCCN3CCCC3)cc12 Show InChI InChI=1S/C26H32N2O2/c1-27-14-13-22(20-7-3-2-4-8-20)23-11-12-26(29)24-10-9-21(19-25(23)24)30-18-17-28-15-5-6-16-28/h2-4,7-12,19,22,27,29H,5-6,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50330791

(CHEMBL1277595 | N-Methyl-4-phenyl-4-(4-(2-(pyrroli...)Show InChI InChI=1S/C23H32N2O/c1-24-15-7-10-23(20-8-3-2-4-9-20)21-11-13-22(14-12-21)26-19-18-25-16-5-6-17-25/h2-4,8-9,11-14,23-24H,5-7,10,15-19H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50330787

(4-(3-(Methylamino)-1-phenylpropyl)-6-(2-(pyrrolidi...)Show SMILES CNCCC(c1ccccc1)c1ccc(O)c2ccc(OCCN3CCCC3)cc12 Show InChI InChI=1S/C26H32N2O2/c1-27-14-13-22(20-7-3-2-4-8-20)23-11-12-26(29)24-10-9-21(19-25(23)24)30-18-17-28-15-5-6-16-28/h2-4,7-12,19,22,27,29H,5-6,13-18H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50330787

(4-(3-(Methylamino)-1-phenylpropyl)-6-(2-(pyrrolidi...)Show SMILES CNCCC(c1ccccc1)c1ccc(O)c2ccc(OCCN3CCCC3)cc12 Show InChI InChI=1S/C26H32N2O2/c1-27-14-13-22(20-7-3-2-4-8-20)23-11-12-26(29)24-10-9-21(19-25(23)24)30-18-17-28-15-5-6-16-28/h2-4,7-12,19,22,27,29H,5-6,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50330786
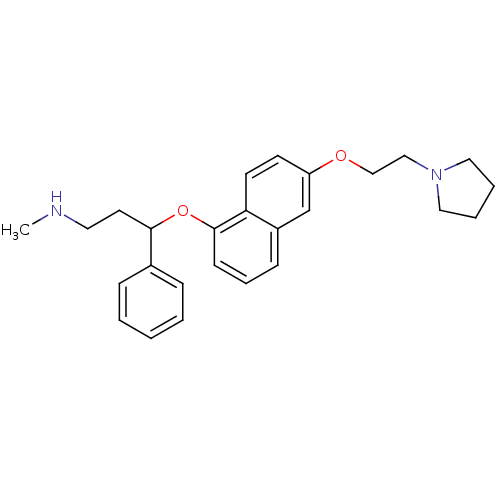
(CHEMBL1277410 | N-Methyl-3-phenyl-3-(6-(2-(pyrroli...)Show InChI InChI=1S/C26H32N2O2/c1-27-15-14-25(21-8-3-2-4-9-21)30-26-11-7-10-22-20-23(12-13-24(22)26)29-19-18-28-16-5-6-17-28/h2-4,7-13,20,25,27H,5-6,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50330789

(CHEMBL1277501 | N1-Methyl-N3-phenyl-N3-(4-(2-(pyrr...)Show InChI InChI=1S/C22H31N3O/c1-23-14-7-17-25(20-8-3-2-4-9-20)21-10-12-22(13-11-21)26-19-18-24-15-5-6-16-24/h2-4,8-13,23H,5-7,14-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50330790

(CHEMBL1277594 | N-Methyl-3-phenyl-3-(4-(2-(pyrroli...)Show InChI InChI=1S/C22H30N2O/c1-23-14-13-22(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-24-15-5-6-16-24/h2-4,7-12,22-23H,5-6,13-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50330791

(CHEMBL1277595 | N-Methyl-4-phenyl-4-(4-(2-(pyrroli...)Show InChI InChI=1S/C23H32N2O/c1-24-15-7-10-23(20-8-3-2-4-9-20)21-11-13-22(14-12-21)26-19-18-25-16-5-6-17-25/h2-4,8-9,11-14,23-24H,5-7,10,15-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50330788

(CHEMBL1277500 | N1-Methyl-N2-phenyl-N2-(4-(2-(pyrr...)Show InChI InChI=1S/C21H29N3O/c1-22-13-16-24(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-23-14-5-6-15-23/h2-4,7-12,22H,5-6,13-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50330788

(CHEMBL1277500 | N1-Methyl-N2-phenyl-N2-(4-(2-(pyrr...)Show InChI InChI=1S/C21H29N3O/c1-22-13-16-24(19-7-3-2-4-8-19)20-9-11-21(12-10-20)25-18-17-23-14-5-6-15-23/h2-4,7-12,22H,5-6,13-18H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50330789

(CHEMBL1277501 | N1-Methyl-N3-phenyl-N3-(4-(2-(pyrr...)Show InChI InChI=1S/C22H31N3O/c1-23-14-7-17-25(20-8-3-2-4-9-20)21-10-12-22(13-11-21)26-19-18-24-15-5-6-16-24/h2-4,8-13,23H,5-7,14-19H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50330786

(CHEMBL1277410 | N-Methyl-3-phenyl-3-(6-(2-(pyrroli...)Show InChI InChI=1S/C26H32N2O2/c1-27-15-14-25(21-8-3-2-4-9-21)30-26-11-7-10-22-20-23(12-13-24(22)26)29-19-18-28-16-5-6-17-28/h2-4,7-13,20,25,27H,5-6,14-19H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from rat H3 receptor |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cells |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
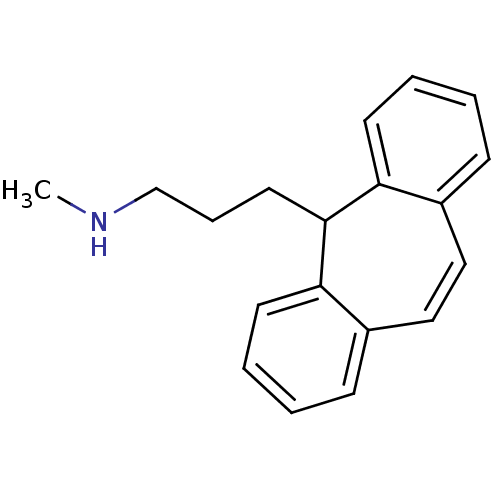
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data