 Found 61 hits Enz. Inhib. hit(s) with all data for entry = 50032573
Found 61 hits Enz. Inhib. hit(s) with all data for entry = 50032573 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331161
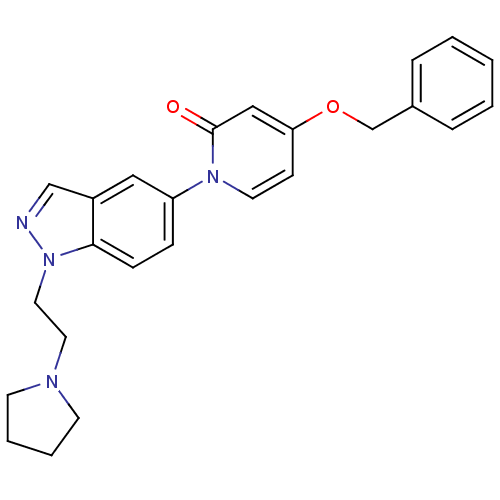
(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331251
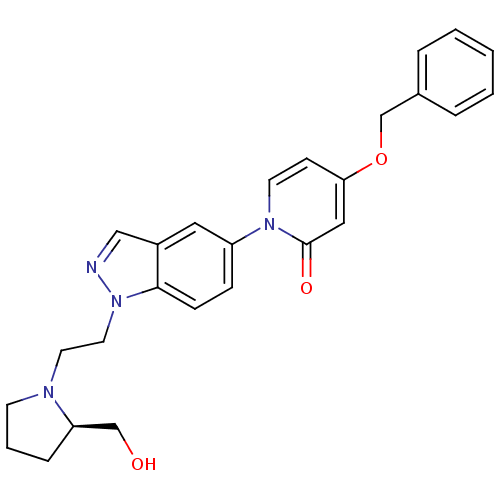
((R)-4-(benzyloxy)-1-(1-(2-(2-(hydroxymethyl)pyrrol...)Show SMILES OC[C@H]1CCCN1CCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O |r| Show InChI InChI=1S/C26H28N4O3/c31-18-23-7-4-11-28(23)13-14-30-25-9-8-22(15-21(25)17-27-30)29-12-10-24(16-26(29)32)33-19-20-5-2-1-3-6-20/h1-3,5-6,8-10,12,15-17,23,31H,4,7,11,13-14,18-19H2/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331242
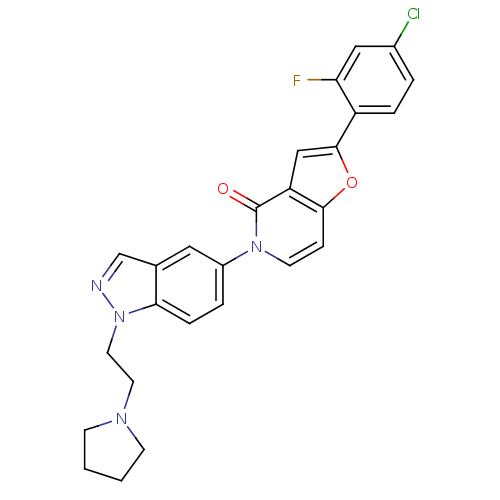
(2-(4-chloro-2-fluorophenyl)-5-(1-(2-(pyrrolidin-1-...)Show SMILES Fc1cc(Cl)ccc1-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C26H22ClFN4O2/c27-18-3-5-20(22(28)14-18)25-15-21-24(34-25)7-10-31(26(21)33)19-4-6-23-17(13-19)16-29-32(23)12-11-30-8-1-2-9-30/h3-7,10,13-16H,1-2,8-9,11-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331245
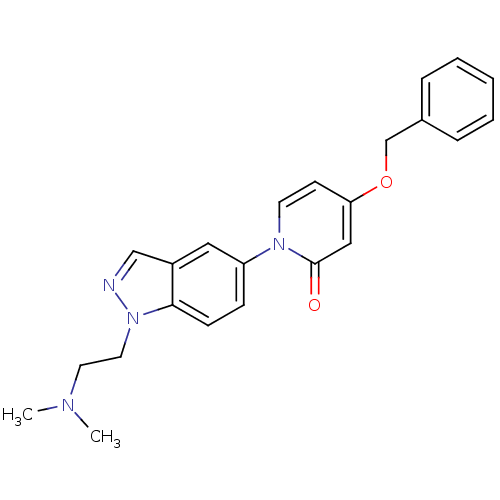
(4-(benzyloxy)-1-(1-(2-(dimethylamino)ethyl)-1H-ind...)Show SMILES CN(C)CCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C23H24N4O2/c1-25(2)12-13-27-22-9-8-20(14-19(22)16-24-27)26-11-10-21(15-23(26)28)29-17-18-6-4-3-5-7-18/h3-11,14-16H,12-13,17H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331234
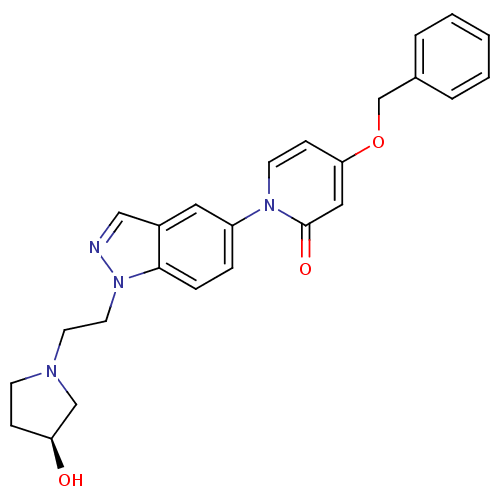
((S)-4-(benzyloxy)-1-(1-(2-(3-hydroxypyrrolidin-1-y...)Show SMILES O[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C25H26N4O3/c30-22-8-10-27(17-22)12-13-29-24-7-6-21(14-20(24)16-26-29)28-11-9-23(15-25(28)31)32-18-19-4-2-1-3-5-19/h1-7,9,11,14-16,22,30H,8,10,12-13,17-18H2/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331236
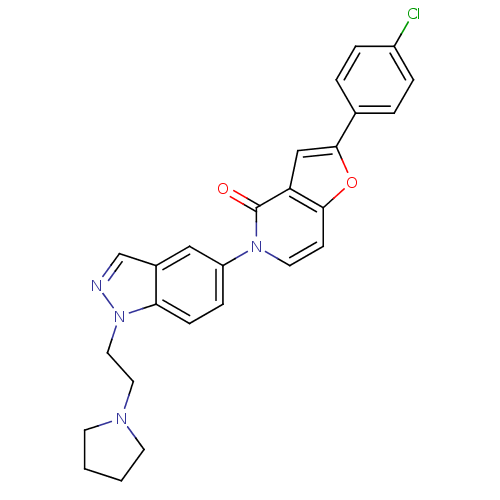
(2-(4-chlorophenyl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)...)Show SMILES Clc1ccc(cc1)-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C26H23ClN4O2/c27-20-5-3-18(4-6-20)25-16-22-24(33-25)9-12-30(26(22)32)21-7-8-23-19(15-21)17-28-31(23)14-13-29-10-1-2-11-29/h3-9,12,15-17H,1-2,10-11,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331239
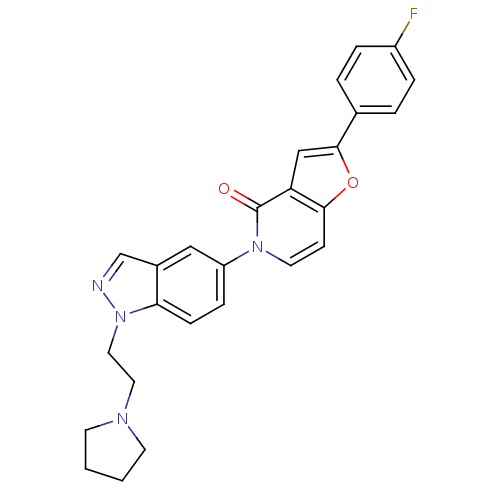
(2-(4-fluorophenyl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)...)Show SMILES Fc1ccc(cc1)-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C26H23FN4O2/c27-20-5-3-18(4-6-20)25-16-22-24(33-25)9-12-30(26(22)32)21-7-8-23-19(15-21)17-28-31(23)14-13-29-10-1-2-11-29/h3-9,12,15-17H,1-2,10-11,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331248
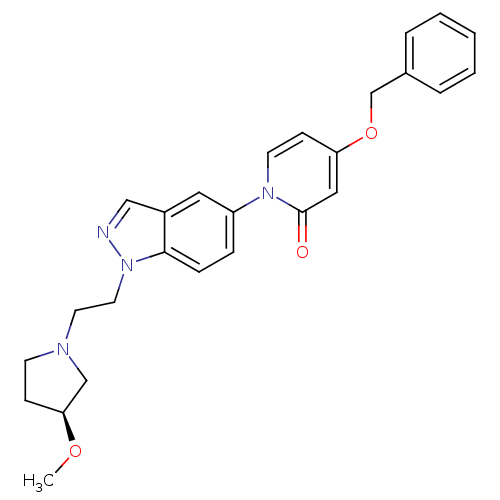
((S)-4-(benzyloxy)-1-(1-(2-(3-methoxypyrrolidin-1-y...)Show SMILES CO[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C26H28N4O3/c1-32-24-9-11-28(18-24)13-14-30-25-8-7-22(15-21(25)17-27-30)29-12-10-23(16-26(29)31)33-19-20-5-3-2-4-6-20/h2-8,10,12,15-17,24H,9,11,13-14,18-19H2,1H3/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331244

(2-phenyl-5-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazo...)Show SMILES O=c1n(ccc2oc(cc12)-c1ccccc1)-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C26H24N4O2/c31-26-22-17-25(19-6-2-1-3-7-19)32-24(22)10-13-29(26)21-8-9-23-20(16-21)18-27-30(23)15-14-28-11-4-5-12-28/h1-3,6-10,13,16-18H,4-5,11-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331252
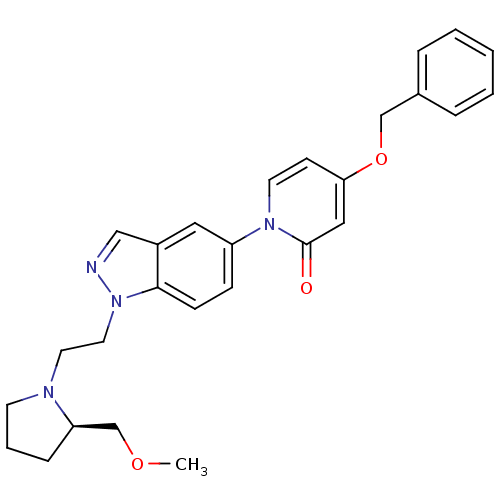
((R)-4-(benzyloxy)-1-(1-(2-(2-(methoxymethyl)pyrrol...)Show SMILES COC[C@H]1CCCN1CCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O |r| Show InChI InChI=1S/C27H30N4O3/c1-33-20-24-8-5-12-29(24)14-15-31-26-10-9-23(16-22(26)18-28-31)30-13-11-25(17-27(30)32)34-19-21-6-3-2-4-7-21/h2-4,6-7,9-11,13,16-18,24H,5,8,12,14-15,19-20H2,1H3/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331249
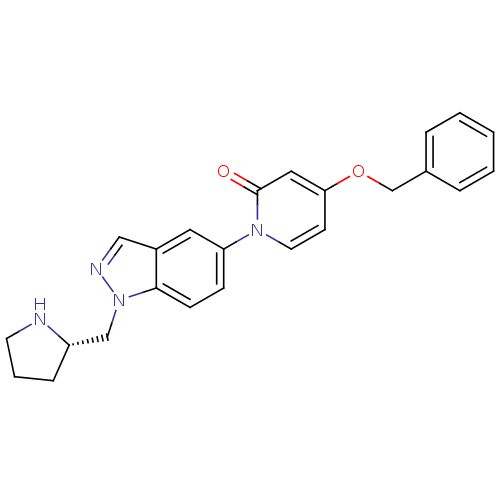
((S)-4-(benzyloxy)-1-(1-(pyrrolidin-2-ylmethyl)-1H-...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(C[C@@H]3CCCN3)ncc2c1 |r| Show InChI InChI=1S/C24H24N4O2/c29-24-14-22(30-17-18-5-2-1-3-6-18)10-12-27(24)21-8-9-23-19(13-21)15-26-28(23)16-20-7-4-11-25-20/h1-3,5-6,8-10,12-15,20,25H,4,7,11,16-17H2/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331246
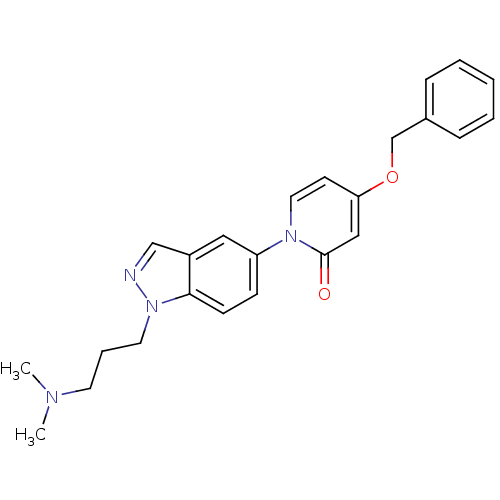
(4-(benzyloxy)-1-(1-(3-(dimethylamino)propyl)-1H-in...)Show SMILES CN(C)CCCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C24H26N4O2/c1-26(2)12-6-13-28-23-10-9-21(15-20(23)17-25-28)27-14-11-22(16-24(27)29)30-18-19-7-4-3-5-8-19/h3-5,7-11,14-17H,6,12-13,18H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331243

(2-(2,4-dichlorophenyl)-5-(1-(2-(pyrrolidin-1-yl)et...)Show SMILES Clc1ccc(-c2cc3c(ccn(-c4ccc5n(CCN6CCCC6)ncc5c4)c3=O)o2)c(Cl)c1 Show InChI InChI=1S/C26H22Cl2N4O2/c27-18-3-5-20(22(28)14-18)25-15-21-24(34-25)7-10-31(26(21)33)19-4-6-23-17(13-19)16-29-32(23)12-11-30-8-1-2-9-30/h3-7,10,13-16H,1-2,8-9,11-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331255
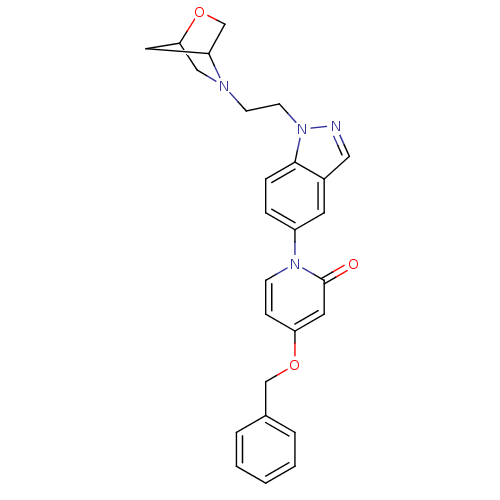
(1-(1-(2-(2-oxa-5-azabicyclo[2.2.1]heptan-5-yl)ethy...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CC4CC3CO4)ncc2c1 |TLB:21:22:25:27.28| Show InChI InChI=1S/C26H26N4O3/c31-26-14-23(32-17-19-4-2-1-3-5-19)8-9-29(26)21-6-7-25-20(12-21)15-27-30(25)11-10-28-16-24-13-22(28)18-33-24/h1-9,12,14-15,22,24H,10-11,13,16-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331238

(4-(benzyloxy)-1-(1-((4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CC3=NCCN3)ncc2c1 |t:23| Show InChI InChI=1S/C23H21N5O2/c29-23-13-20(30-16-17-4-2-1-3-5-17)8-11-27(23)19-6-7-21-18(12-19)14-26-28(21)15-22-24-9-10-25-22/h1-8,11-14H,9-10,15-16H2,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331256
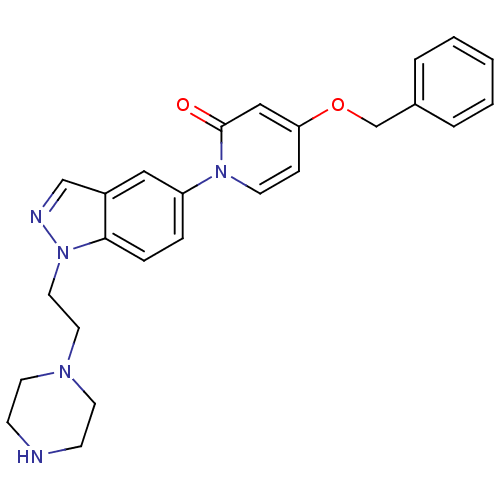
(4-(benzyloxy)-1-(1-(2-(piperazin-1-yl)ethyl)-1H-in...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCNCC3)ncc2c1 Show InChI InChI=1S/C25H27N5O2/c31-25-17-23(32-19-20-4-2-1-3-5-20)8-11-29(25)22-6-7-24-21(16-22)18-27-30(24)15-14-28-12-9-26-10-13-28/h1-8,11,16-18,26H,9-10,12-15,19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331235
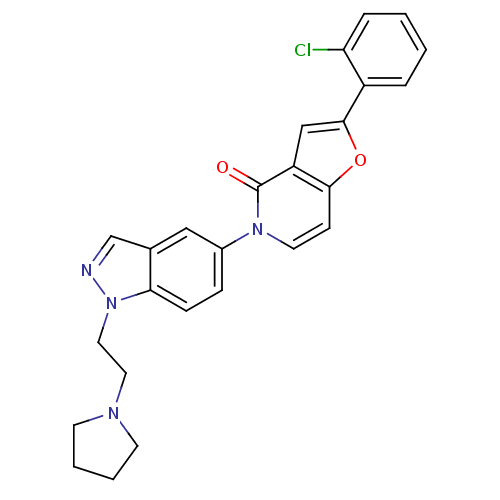
(2-(2-chlorophenyl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)...)Show SMILES Clc1ccccc1-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C26H23ClN4O2/c27-22-6-2-1-5-20(22)25-16-21-24(33-25)9-12-30(26(21)32)19-7-8-23-18(15-19)17-28-31(23)14-13-29-10-3-4-11-29/h1-2,5-9,12,15-17H,3-4,10-11,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331253

(4-(benzyloxy)-1-(1-((1-methyl-4,5-dihydro-1H-imida...)Show SMILES CN1CCN=C1Cn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O |c:4| Show InChI InChI=1S/C24H23N5O2/c1-27-12-10-25-23(27)16-29-22-8-7-20(13-19(22)15-26-29)28-11-9-21(14-24(28)30)31-17-18-5-3-2-4-6-18/h2-9,11,13-15H,10,12,16-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331250
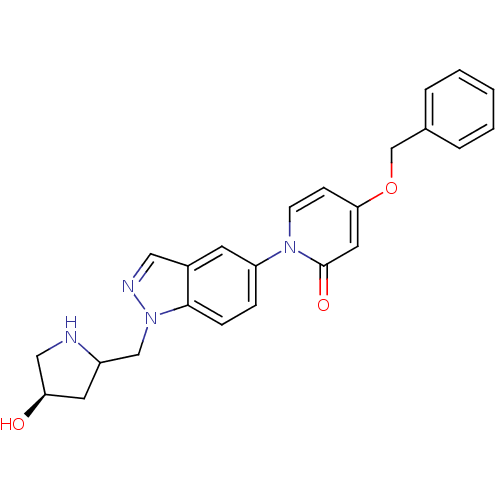
(4-(benzyloxy)-1-(1-(((4R)-4-hydroxypyrrolidin-2-yl...)Show SMILES O[C@H]1CNC(Cn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C24H24N4O3/c29-21-11-19(25-14-21)15-28-23-7-6-20(10-18(23)13-26-28)27-9-8-22(12-24(27)30)31-16-17-4-2-1-3-5-17/h1-10,12-13,19,21,25,29H,11,14-16H2/t19?,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331247
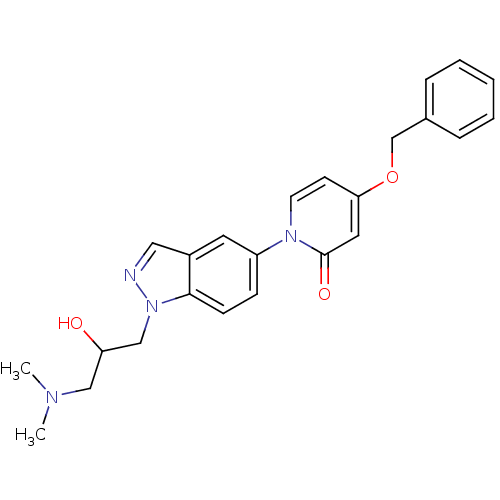
(4-(benzyloxy)-1-(1-(3-(dimethylamino)-2-hydroxypro...)Show SMILES CN(C)CC(O)Cn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C24H26N4O3/c1-26(2)15-21(29)16-28-23-9-8-20(12-19(23)14-25-28)27-11-10-22(13-24(27)30)31-17-18-6-4-3-5-7-18/h3-14,21,29H,15-17H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331241

(2-(3-chlorophenyl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)...)Show SMILES Clc1cccc(c1)-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C26H23ClN4O2/c27-20-5-3-4-18(14-20)25-16-22-24(33-25)8-11-30(26(22)32)21-6-7-23-19(15-21)17-28-31(23)13-12-29-9-1-2-10-29/h3-8,11,14-17H,1-2,9-10,12-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331254
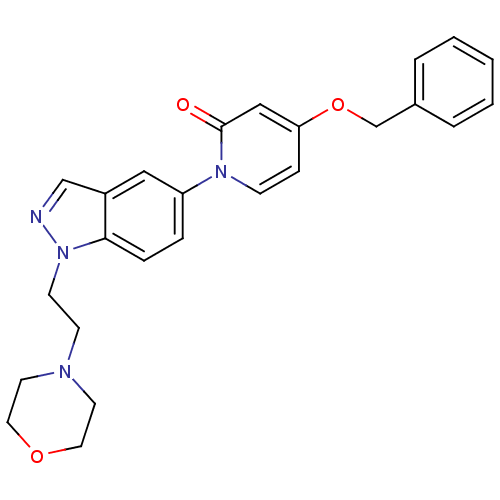
(4-(benzyloxy)-1-(1-(2-morpholinoethyl)-1H-indazol-...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCOCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O3/c30-25-17-23(32-19-20-4-2-1-3-5-20)8-9-28(25)22-6-7-24-21(16-22)18-26-29(24)11-10-27-12-14-31-15-13-27/h1-9,16-18H,10-15,19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331240
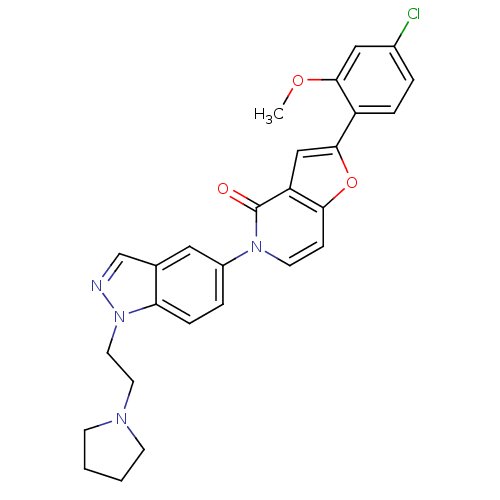
(2-(4-chloro-2-methoxyphenyl)-5-(1-(2-(pyrrolidin-1...)Show SMILES COc1cc(Cl)ccc1-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C27H25ClN4O3/c1-34-25-15-19(28)4-6-21(25)26-16-22-24(35-26)8-11-31(27(22)33)20-5-7-23-18(14-20)17-29-32(23)13-12-30-9-2-3-10-30/h4-8,11,14-17H,2-3,9-10,12-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331237
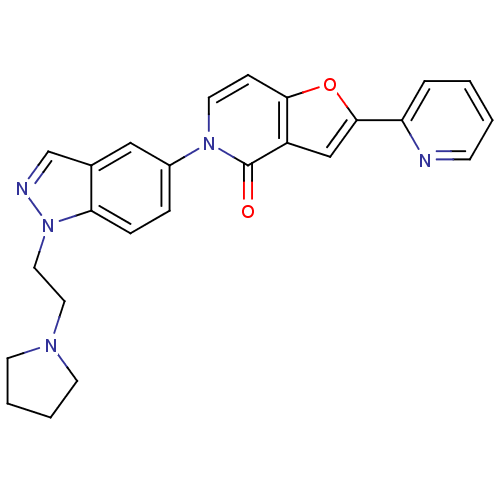
(2-(pyridin-2-yl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)-1...)Show SMILES O=c1n(ccc2oc(cc12)-c1ccccn1)-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H23N5O2/c31-25-20-16-24(21-5-1-2-9-26-21)32-23(20)8-12-29(25)19-6-7-22-18(15-19)17-27-30(22)14-13-28-10-3-4-11-28/h1-2,5-9,12,15-17H,3-4,10-11,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331233
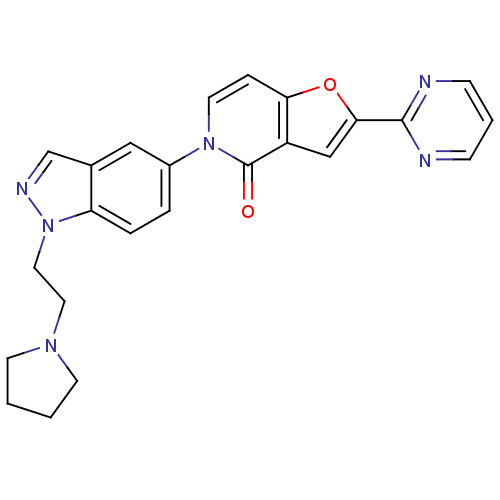
(2-(pyrimidin-2-yl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)...)Show SMILES O=c1n(ccc2oc(cc12)-c1ncccn1)-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C24H22N6O2/c31-24-19-15-22(23-25-7-3-8-26-23)32-21(19)6-11-29(24)18-4-5-20-17(14-18)16-27-30(20)13-12-28-9-1-2-10-28/h3-8,11,14-16H,1-2,9-10,12-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331232
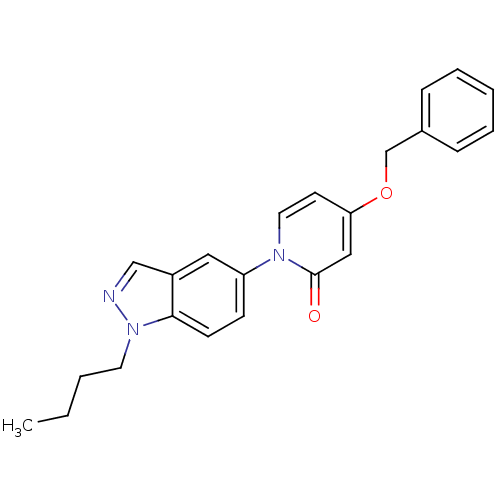
(4-(benzyloxy)-1-(1-butyl-1H-indazol-5-yl)pyridin-2...)Show InChI InChI=1S/C23H23N3O2/c1-2-3-12-26-22-10-9-20(14-19(22)16-24-26)25-13-11-21(15-23(25)27)28-17-18-7-5-4-6-8-18/h4-11,13-16H,2-3,12,17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indazol-5-yl)pyridin-2(1H)-one from MCH-1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Antagonistic activity at MCH-1 receptor expressed in CHO-K1 cell assessed as inhibition of MCH-induced calcium release |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50331236

(2-(4-chlorophenyl)-5-(1-(2-(pyrrolidin-1-yl)ethyl)...)Show SMILES Clc1ccc(cc1)-c1cc2c(ccn(-c3ccc4n(CCN5CCCC5)ncc4c3)c2=O)o1 Show InChI InChI=1S/C26H23ClN4O2/c27-20-5-3-18(4-6-20)25-16-22-24(33-25)9-12-30(26(22)32)21-7-8-23-19(15-21)17-28-31(23)14-13-29-10-1-2-11-29/h3-9,12,15-17H,1-2,10-11,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Antagonistic activity at MCH-1 receptor expressed in CHO-K1 cell assessed as inhibition of MCH-induced calcium release |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331252

((R)-4-(benzyloxy)-1-(1-(2-(2-(methoxymethyl)pyrrol...)Show SMILES COC[C@H]1CCCN1CCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O |r| Show InChI InChI=1S/C27H30N4O3/c1-33-20-24-8-5-12-29(24)14-15-31-26-10-9-23(16-22(26)18-28-31)30-13-11-25(17-27(30)32)34-19-21-6-3-2-4-7-21/h2-4,6-7,9-11,13,16-18,24H,5,8,12,14-15,19-20H2,1H3/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331246

(4-(benzyloxy)-1-(1-(3-(dimethylamino)propyl)-1H-in...)Show SMILES CN(C)CCCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C24H26N4O2/c1-26(2)12-6-13-28-23-10-9-21(15-20(23)17-25-28)27-14-11-22(16-24(27)29)30-18-19-7-4-3-5-8-19/h3-5,7-11,14-17H,6,12-13,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331248

((S)-4-(benzyloxy)-1-(1-(2-(3-methoxypyrrolidin-1-y...)Show SMILES CO[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C26H28N4O3/c1-32-24-9-11-28(18-24)13-14-30-25-8-7-22(15-21(25)17-27-30)29-12-10-23(16-26(29)31)33-19-20-5-3-2-4-6-20/h2-8,10,12,15-17,24H,9,11,13-14,18-19H2,1H3/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331245

(4-(benzyloxy)-1-(1-(2-(dimethylamino)ethyl)-1H-ind...)Show SMILES CN(C)CCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O Show InChI InChI=1S/C23H24N4O2/c1-25(2)12-13-27-22-9-8-20(14-19(22)16-24-27)26-11-10-21(15-23(26)28)29-17-18-6-4-3-5-7-18/h3-11,14-16H,12-13,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331249

((S)-4-(benzyloxy)-1-(1-(pyrrolidin-2-ylmethyl)-1H-...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(C[C@@H]3CCCN3)ncc2c1 |r| Show InChI InChI=1S/C24H24N4O2/c29-24-14-22(30-17-18-5-2-1-3-6-18)10-12-27(24)21-8-9-23-19(13-21)15-26-28(23)16-20-7-4-11-25-20/h1-3,5-6,8-10,12-15,20,25H,4,7,11,16-17H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331251

((R)-4-(benzyloxy)-1-(1-(2-(2-(hydroxymethyl)pyrrol...)Show SMILES OC[C@H]1CCCN1CCn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O |r| Show InChI InChI=1S/C26H28N4O3/c31-18-23-7-4-11-28(23)13-14-30-25-9-8-22(15-21(25)17-27-30)29-12-10-24(16-26(29)32)33-19-20-5-2-1-3-6-20/h1-3,5-6,8-10,12,15-17,23,31H,4,7,11,13-14,18-19H2/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331253

(4-(benzyloxy)-1-(1-((1-methyl-4,5-dihydro-1H-imida...)Show SMILES CN1CCN=C1Cn1ncc2cc(ccc12)-n1ccc(OCc2ccccc2)cc1=O |c:4| Show InChI InChI=1S/C24H23N5O2/c1-27-12-10-25-23(27)16-29-22-8-7-20(13-19(22)15-26-29)28-11-9-21(14-24(28)30)31-17-18-5-3-2-4-6-18/h2-9,11,13-15H,10,12,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50331234

((S)-4-(benzyloxy)-1-(1-(2-(3-hydroxypyrrolidin-1-y...)Show SMILES O[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C25H26N4O3/c30-22-8-10-27(17-22)12-13-29-24-7-6-21(14-20(24)16-26-29)28-11-9-23(15-25(28)31)32-18-19-4-2-1-3-5-19/h1-7,9,11,14-16,22,30H,8,10,12-13,17-18H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331238

(4-(benzyloxy)-1-(1-((4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CC3=NCCN3)ncc2c1 |t:23| Show InChI InChI=1S/C23H21N5O2/c29-23-13-20(30-16-17-4-2-1-3-5-17)8-11-27(23)19-6-7-21-18(12-19)14-26-28(21)15-22-24-9-10-25-22/h1-8,11-14H,9-10,15-16H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331234

((S)-4-(benzyloxy)-1-(1-(2-(3-hydroxypyrrolidin-1-y...)Show SMILES O[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C25H26N4O3/c30-22-8-10-27(17-22)12-13-29-24-7-6-21(14-20(24)16-26-29)28-11-9-23(15-25(28)31)32-18-19-4-2-1-3-5-19/h1-7,9,11,14-16,22,30H,8,10,12-13,17-18H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells by mini-patch clamp assay |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50331238

(4-(benzyloxy)-1-(1-((4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CC3=NCCN3)ncc2c1 |t:23| Show InChI InChI=1S/C23H21N5O2/c29-23-13-20(30-16-17-4-2-1-3-5-17)8-11-27(23)19-6-7-21-18(12-19)14-26-28(21)15-22-24-9-10-25-22/h1-8,11-14H,9-10,15-16H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50331234

((S)-4-(benzyloxy)-1-(1-(2-(3-hydroxypyrrolidin-1-y...)Show SMILES O[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C25H26N4O3/c30-22-8-10-27(17-22)12-13-29-24-7-6-21(14-20(24)16-26-29)28-11-9-23(15-25(28)31)32-18-19-4-2-1-3-5-19/h1-7,9,11,14-16,22,30H,8,10,12-13,17-18H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50331238

(4-(benzyloxy)-1-(1-((4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CC3=NCCN3)ncc2c1 |t:23| Show InChI InChI=1S/C23H21N5O2/c29-23-13-20(30-16-17-4-2-1-3-5-17)8-11-27(23)19-6-7-21-18(12-19)14-26-28(21)15-22-24-9-10-25-22/h1-8,11-14H,9-10,15-16H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50331234

((S)-4-(benzyloxy)-1-(1-(2-(3-hydroxypyrrolidin-1-y...)Show SMILES O[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C25H26N4O3/c30-22-8-10-27(17-22)12-13-29-24-7-6-21(14-20(24)16-26-29)28-11-9-23(15-25(28)31)32-18-19-4-2-1-3-5-19/h1-7,9,11,14-16,22,30H,8,10,12-13,17-18H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50331238

(4-(benzyloxy)-1-(1-((4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CC3=NCCN3)ncc2c1 |t:23| Show InChI InChI=1S/C23H21N5O2/c29-23-13-20(30-16-17-4-2-1-3-5-17)8-11-27(23)19-6-7-21-18(12-19)14-26-28(21)15-22-24-9-10-25-22/h1-8,11-14H,9-10,15-16H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50331234

((S)-4-(benzyloxy)-1-(1-(2-(3-hydroxypyrrolidin-1-y...)Show SMILES O[C@H]1CCN(CCn2ncc3cc(ccc23)-n2ccc(OCc3ccccc3)cc2=O)C1 |r| Show InChI InChI=1S/C25H26N4O3/c30-22-8-10-27(17-22)12-13-29-24-7-6-21(14-20(24)16-26-29)28-11-9-23(15-25(28)31)32-18-19-4-2-1-3-5-19/h1-7,9,11,14-16,22,30H,8,10,12-13,17-18H2/t22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50331161

(4-(benzyloxy)-1-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-i...)Show SMILES O=c1cc(OCc2ccccc2)ccn1-c1ccc2n(CCN3CCCC3)ncc2c1 Show InChI InChI=1S/C25H26N4O2/c30-25-17-23(31-19-20-6-2-1-3-7-20)10-13-28(25)22-8-9-24-21(16-22)18-26-29(24)15-14-27-11-4-5-12-27/h1-3,6-10,13,16-18H,4-5,11-12,14-15,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 7015-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.039
BindingDB Entry DOI: 10.7270/Q2P55NSM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data