

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331736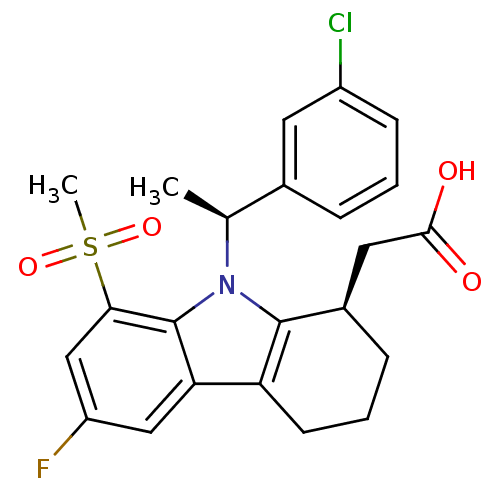 (2-((R)-9-((S)-1-(3-chlorophenyl)ethyl)-6-fluoro-8-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331738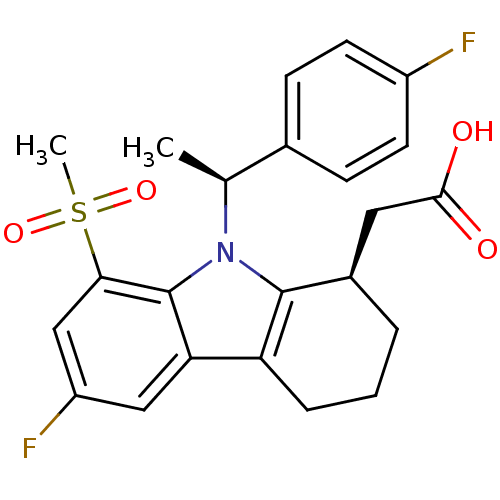 (2-((R)-6-fluoro-9-((S)-1-(4-fluorophenyl)ethyl)-8-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331733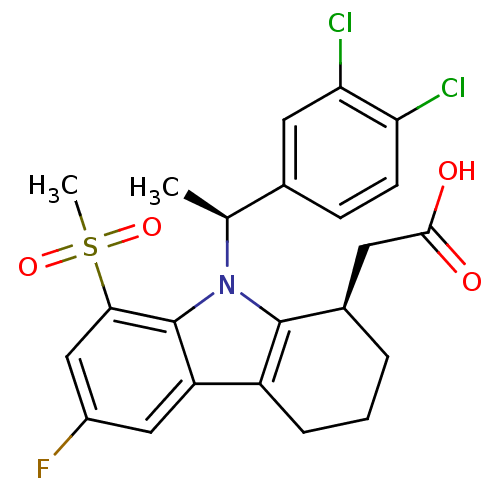 (2-((R)-9-((S)-1-(3,4-dichlorophenyl)ethyl)-6-fluor...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331742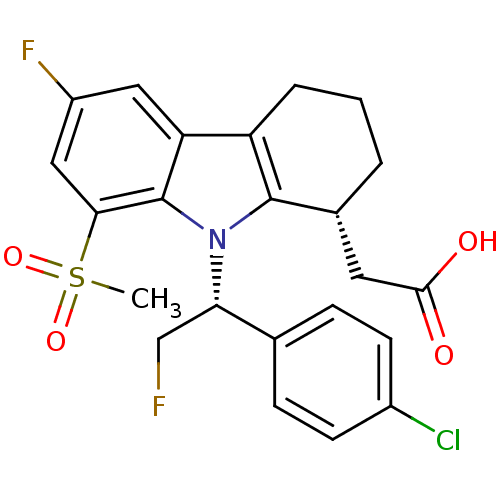 (2-((R)-9-((R)-1-(4-chlorophenyl)-2-fluoroethyl)-6-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331735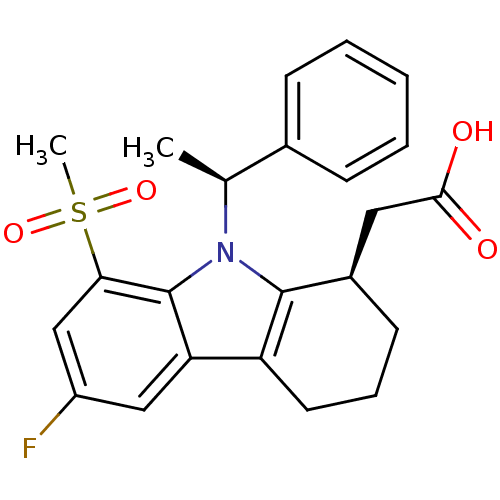 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-phenyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331732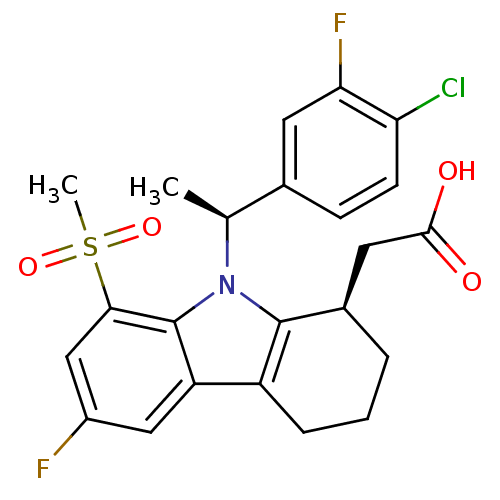 (2-((R)-9-((S)-1-(4-chloro-3-fluorophenyl)ethyl)-6-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331739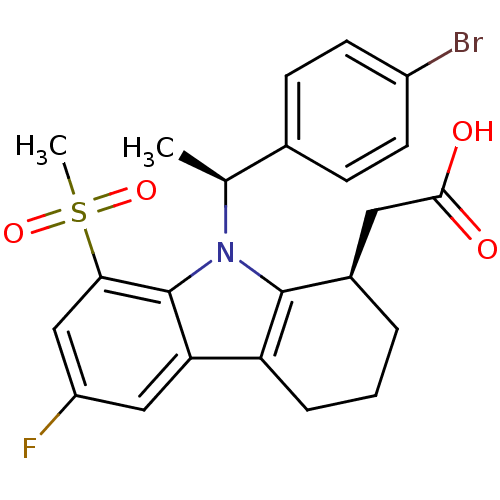 (2-((R)-9-((S)-1-(4-bromophenyl)ethyl)-6-fluoro-8-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331743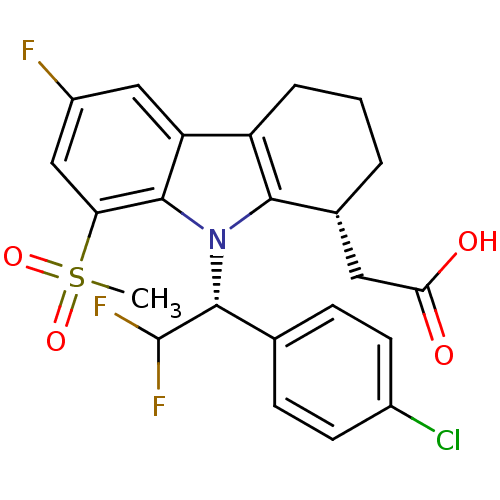 (2-((R)-9-((R)-1-(4-chlorophenyl)-2,2-difluoroethyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331734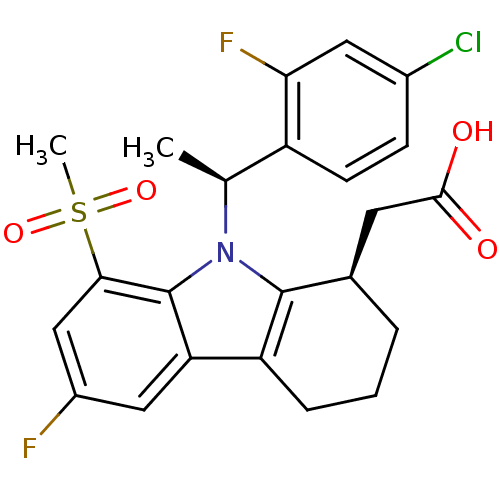 (2-((R)-9-((S)-1-(4-chloro-2-fluorophenyl)ethyl)-6-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331737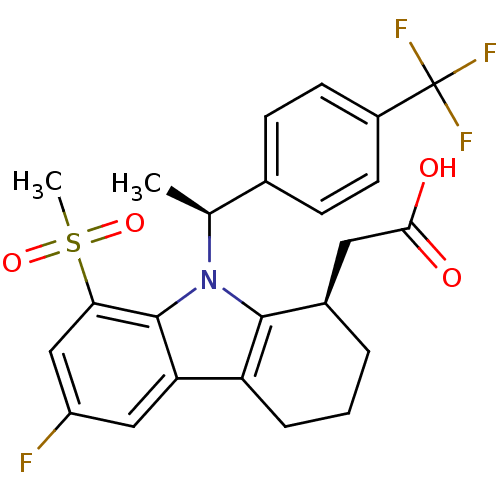 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331740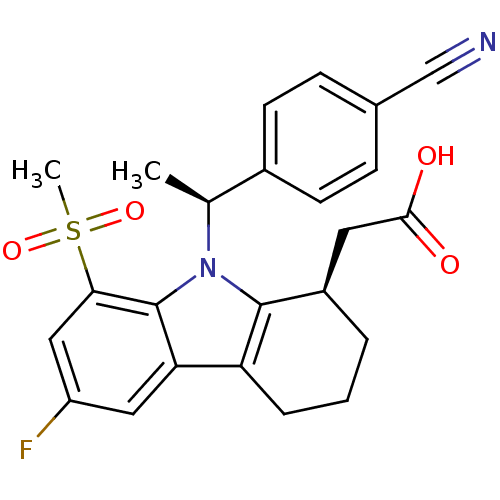 (2-((R)-9-((S)-1-(4-cyanophenyl)ethyl)-6-fluoro-8-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331731 (2-((R)-9-((S)-1-(4-chlorophenyl)ethyl)-6-fluoro-8-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331730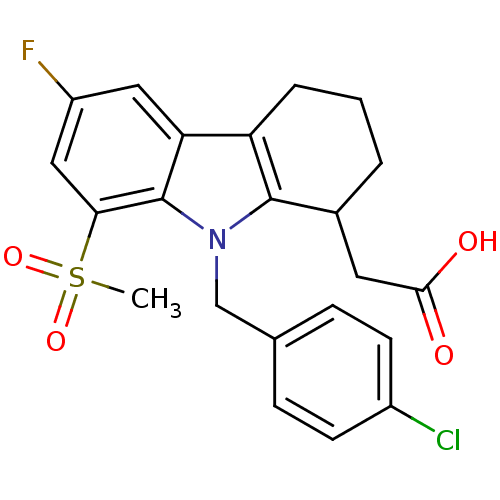 ((+/-)-2-(9-(4-chlorobenzyl)-6-fluoro-8-(methylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331729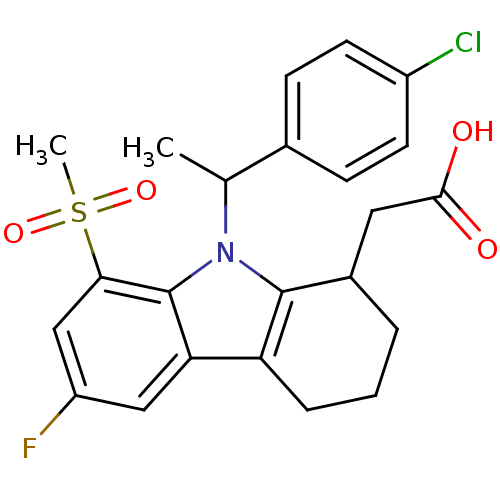 (2-(9-(1-(4-chlorophenyl)ethyl)-6-fluoro-8-(methyls...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331729 (2-(9-(1-(4-chlorophenyl)ethyl)-6-fluoro-8-(methyls...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331730 ((+/-)-2-(9-(4-chlorobenzyl)-6-fluoro-8-(methylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331741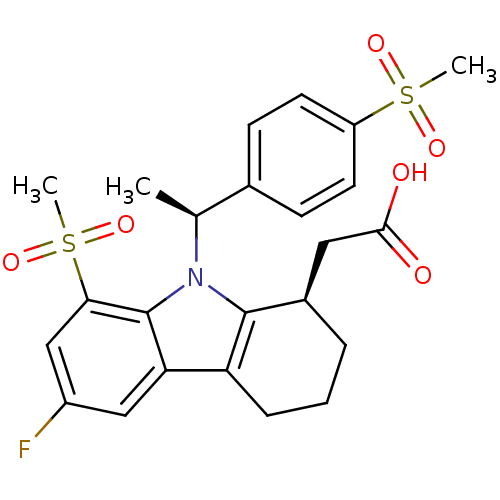 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(me...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331740 (2-((R)-9-((S)-1-(4-cyanophenyl)ethyl)-6-fluoro-8-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331738 (2-((R)-6-fluoro-9-((S)-1-(4-fluorophenyl)ethyl)-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331739 (2-((R)-9-((S)-1-(4-bromophenyl)ethyl)-6-fluoro-8-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331733 (2-((R)-9-((S)-1-(3,4-dichlorophenyl)ethyl)-6-fluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331742 (2-((R)-9-((R)-1-(4-chlorophenyl)-2-fluoroethyl)-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331743 (2-((R)-9-((R)-1-(4-chlorophenyl)-2,2-difluoroethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331731 (2-((R)-9-((S)-1-(4-chlorophenyl)ethyl)-6-fluoro-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331732 (2-((R)-9-((S)-1-(4-chloro-3-fluorophenyl)ethyl)-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331734 (2-((R)-9-((S)-1-(4-chloro-2-fluorophenyl)ethyl)-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331736 (2-((R)-9-((S)-1-(3-chlorophenyl)ethyl)-6-fluoro-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 292 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 338 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331735 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to prostanoid receptor EP3 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331741 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid TP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to prostanoid receptor EP2 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to prostanoid receptor FP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to prostanoid receptor EP1 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to prostanoid receptor EP4 receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to prostanoid receptor IP receptor | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at prostanoid DP1 receptor in human platelet assessed as inhibition of PGD2 induced accumulation of cAMP | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50331737 (2-((R)-6-fluoro-8-(methylsulfonyl)-9-((S)-1-(4-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at TP receptor in human platelet assessed as thromboxane A2- induced platelet aggregation | Bioorg Med Chem Lett 20: 7462-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.018 BindingDB Entry DOI: 10.7270/Q2PZ5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||