

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50332799 (CHEMBL1629796 | N-(3-Chloro-10H-phenothiazin-10-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50332798 (CHEMBL1629795 | N-(3-Chloro-10H-phenothiazin-10-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50332797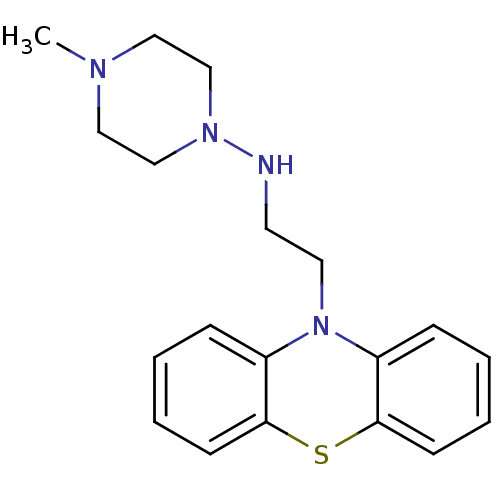 (CHEMBL1629794 | N-[2-(10H-Phenothiazin-10-yl)ethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50332802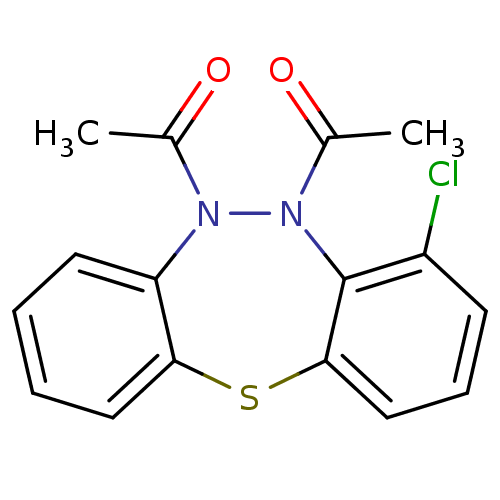 (1,1'-(4-Chlorodibenzo[b,f][1,4,5]thiadiazepine-5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50332797 (CHEMBL1629794 | N-[2-(10H-Phenothiazin-10-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50332798 (CHEMBL1629795 | N-(3-Chloro-10H-phenothiazin-10-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50332800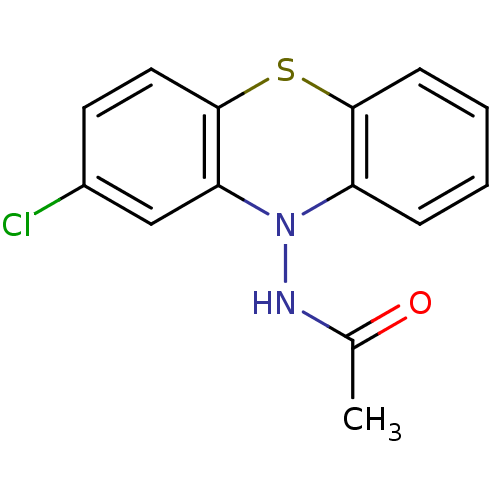 (CHEMBL1629797 | N-(2-Chloro-10H-phenothiazin-10-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50332801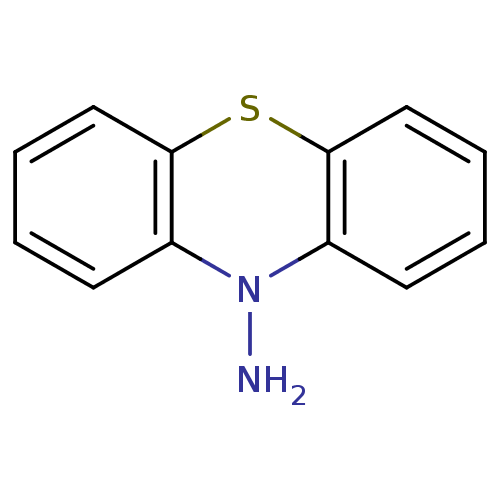 (10H-Phenothiazin-10-amine | CHEMBL1629798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50332800 (CHEMBL1629797 | N-(2-Chloro-10H-phenothiazin-10-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50332801 (10H-Phenothiazin-10-amine | CHEMBL1629798) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50332802 (1,1'-(4-Chlorodibenzo[b,f][1,4,5]thiadiazepine-5,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50332799 (CHEMBL1629796 | N-(3-Chloro-10H-phenothiazin-10-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||