

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic receptor M1 (Bos taurus) | BDBM50010096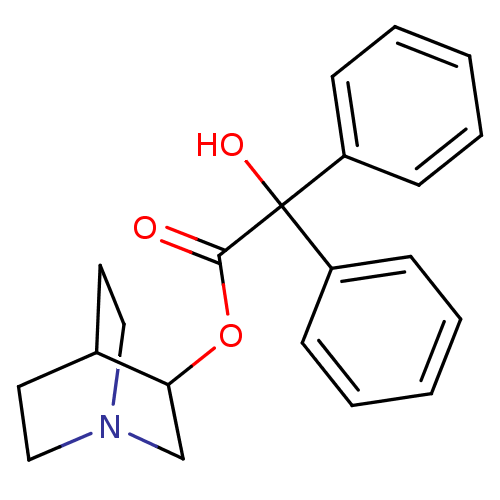 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50403547 (ATROPEN | ATROPINE) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50296314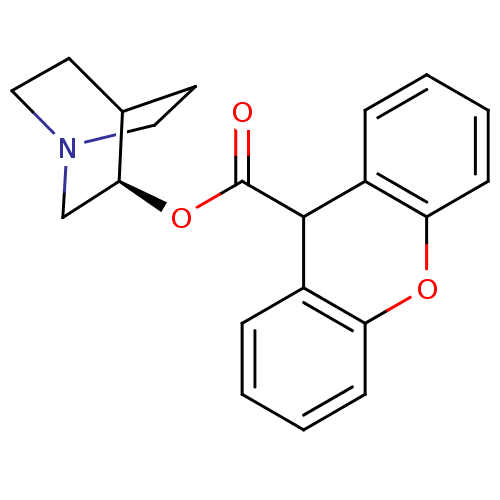 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50010096 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50405720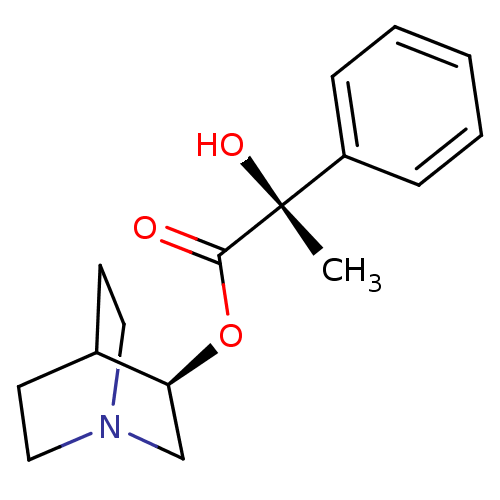 (CHEMBL2115342) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the phosphatidyl inositol turnover at Muscarinic acetylcholine receptor M1 in rat cortex | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50367742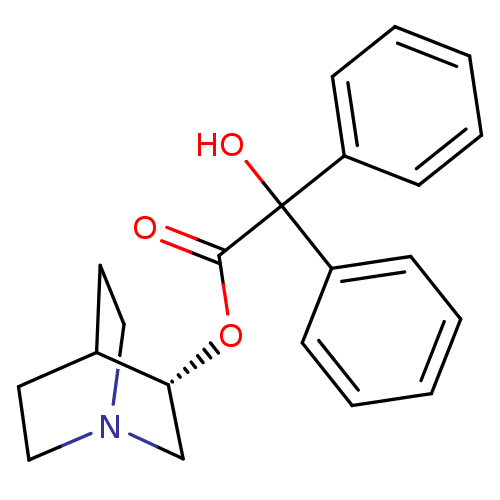 (CHEMBL1788199) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50010096 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50405717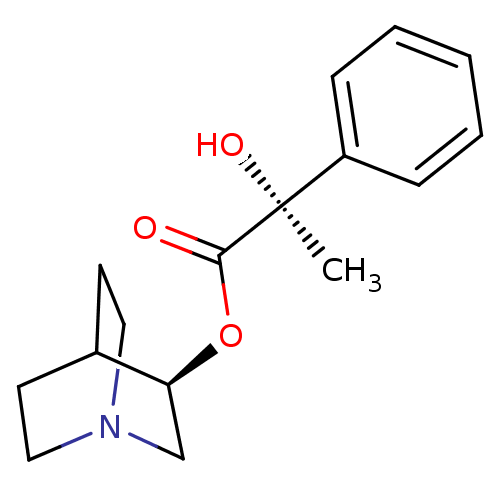 (CHEMBL2115341) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Pseudo hill coefficient at Muscarinic acetylcholine receptor M1 | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50367741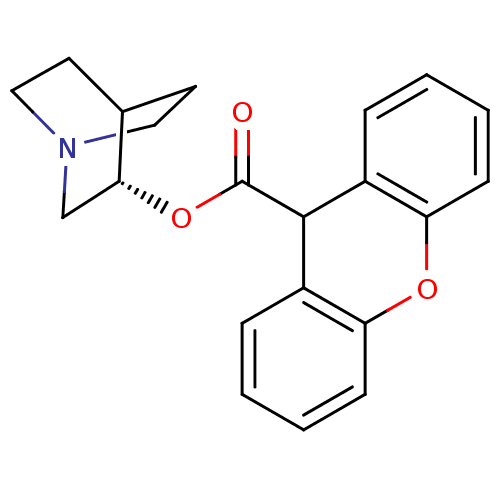 (CHEMBL1788286) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50367742 (CHEMBL1788199) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405720 (CHEMBL2115342) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50405718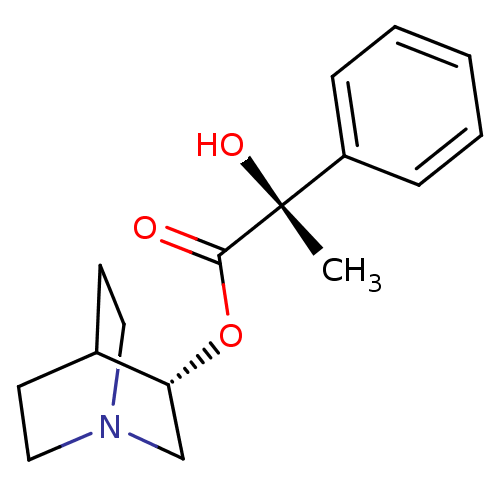 (CHEMBL2114396) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50367741 (CHEMBL1788286) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the phosphatidyl inositol turnover at Muscarinic acetylcholine receptor M1 in rat cortex | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405717 (CHEMBL2115341) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50367741 (CHEMBL1788286) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50367742 (CHEMBL1788199) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405720 (CHEMBL2115342) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50405719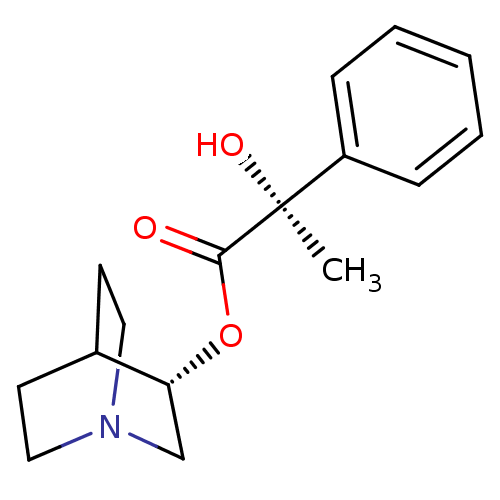 (CHEMBL2114395) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405718 (CHEMBL2114396) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 934 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405719 (CHEMBL2114395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405717 (CHEMBL2115341) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50367741 (CHEMBL1788286) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405719 (CHEMBL2114395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50405718 (CHEMBL2114396) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||