 Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50033043
Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50033043 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338686

((R/S)-3-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C22H23ClN4O3S/c1-24-19-7-2-13-10-14(3-5-16(13)19)27-9-8-20(22(27)28)26-31(29,30)15-4-6-17-18(23)12-25-21(17)11-15/h3-6,10-12,19-20,24-26H,2,7-9H2,1H3/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338689

((R/S)-3-chloro-N-((3S)-1-(1-(dimethylamino)-2,3-di...)Show SMILES CN(C)C1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C23H25ClN4O3S/c1-27(2)22-8-3-14-11-15(4-6-17(14)22)28-10-9-20(23(28)29)26-32(30,31)16-5-7-18-19(24)13-25-21(18)12-16/h4-7,11-13,20,22,25-26H,3,8-10H2,1-2H3/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338678
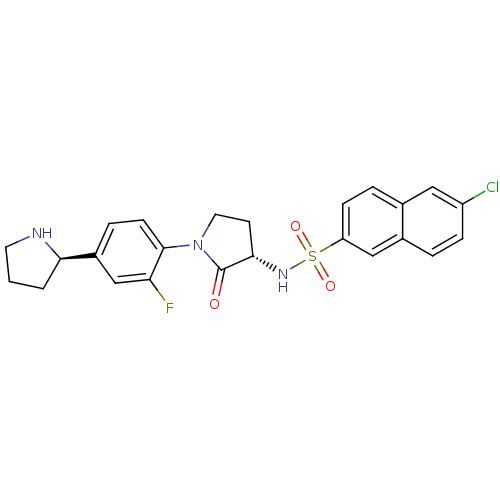
(6-CHLORO-N-((3S)-2-OXO-1-{4-[(2R)-2--PYRROLIDINYL]...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)[C@H]1CCCN1 |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-22-9-11-29(24(22)30)23-8-5-17(14-20(23)26)21-2-1-10-27-21/h3-8,12-14,21-22,27-28H,1-2,9-11H2/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306134
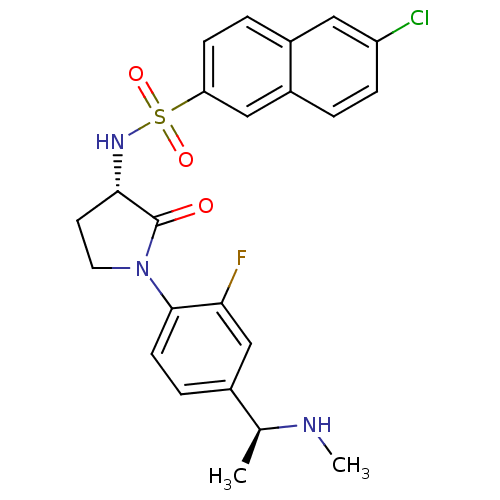
(6-chloro-N-((S)-1-(2-fluoro-4-((S)-1-(methylamino)...)Show SMILES CN[C@@H](C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H23ClFN3O3S/c1-14(26-2)15-5-8-22(20(25)13-15)28-10-9-21(23(28)29)27-32(30,31)19-7-4-16-11-18(24)6-3-17(16)12-19/h3-8,11-14,21,26-27H,9-10H2,1-2H3/t14-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338691

(6-CHLORO-N-[(3S)-1-[(1S)-1-DIMETHYLAMINO-2,3-DIHYD...)Show SMILES CN(C)[C@H]1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-28(2)24-10-5-18-14-20(7-9-22(18)24)29-12-11-23(25(29)30)27-33(31,32)21-8-4-16-13-19(26)6-3-17(16)15-21/h3-4,6-9,13-15,23-24,27H,5,10-12H2,1-2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338677

(6-CHLORO-N-((3S)-2-OXO-1-{4-[(2S)-2-PYRROLIDINYL]P...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-22-9-11-29(24(22)30)23-8-5-17(14-20(23)26)21-2-1-10-27-21/h3-8,12-14,21-22,27-28H,1-2,9-11H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338682
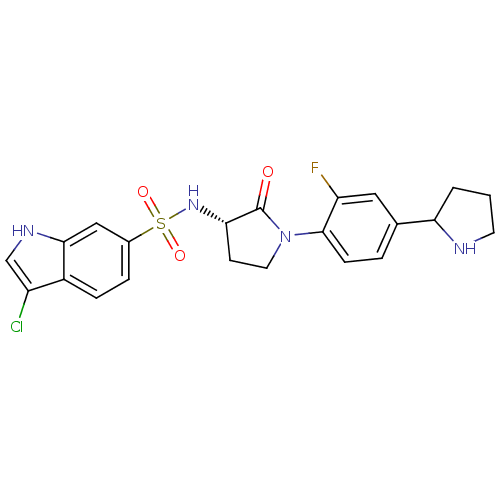
((R/S)-3-chloro-N-((3S)-1-(2-fluoro-4-(pyrrolidin-2...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O)C1CCCN1 |r| Show InChI InChI=1S/C22H22ClFN4O3S/c23-16-12-26-20-11-14(4-5-15(16)20)32(30,31)27-19-7-9-28(22(19)29)21-6-3-13(10-17(21)24)18-2-1-8-25-18/h3-6,10-12,18-19,25-27H,1-2,7-9H2/t18?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338680
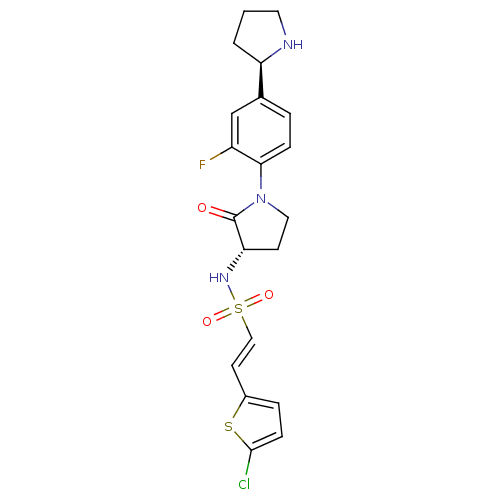
((E)-2-(5-chlorothiophen-2-yl)-N-((S)-1-(2-fluoro-4...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)[C@H]1CCCN1 |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-19-6-4-14(29-19)8-11-30(27,28)24-17-7-10-25(20(17)26)18-5-3-13(12-15(18)22)16-2-1-9-23-16/h3-6,8,11-12,16-17,23-24H,1-2,7,9-10H2/b11-8+/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338690

(6-CHLORO-N-{(3S)-1-[(1S)-1-(DIMETHYLAMINO)-2,3-DIH...)Show SMILES CN(C)[C@@H]1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-28(2)24-10-5-18-14-20(7-9-22(18)24)29-12-11-23(25(29)30)27-33(31,32)21-8-4-16-13-19(26)6-3-17(16)15-21/h3-4,6-9,13-15,23-24,27H,5,10-12H2,1-2H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338688
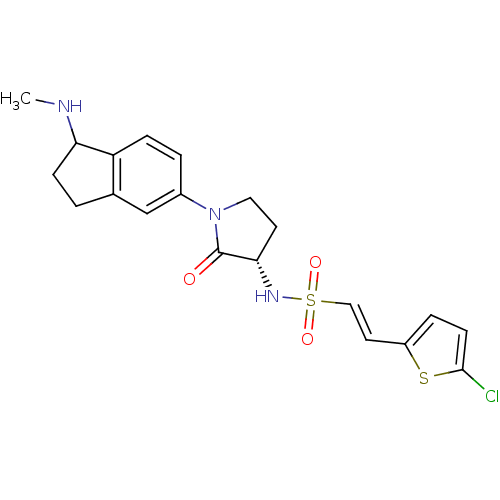
((R/S)-(E)-2-(5-chlorothiophen-2-yl)-N-((3S)-1-(1-(...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H22ClN3O3S2/c1-22-17-6-2-13-12-14(3-5-16(13)17)24-10-8-18(20(24)25)23-29(26,27)11-9-15-4-7-19(21)28-15/h3-5,7,9,11-12,17-18,22-23H,2,6,8,10H2,1H3/b11-9+/t17?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338692

((R/S)-(E)-2-(5-chlorothiophen-2-yl)-N-((3S)-1-(1-(...)Show SMILES CN(C)C1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C21H24ClN3O3S2/c1-24(2)19-7-3-14-13-15(4-6-17(14)19)25-11-9-18(21(25)26)23-30(27,28)12-10-16-5-8-20(22)29-16/h4-6,8,10,12-13,18-19,23H,3,7,9,11H2,1-2H3/b12-10+/t18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338677

(6-CHLORO-N-((3S)-2-OXO-1-{4-[(2S)-2-PYRROLIDINYL]P...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-22-9-11-29(24(22)30)23-8-5-17(14-20(23)26)21-2-1-10-27-21/h3-8,12-14,21-22,27-28H,1-2,9-11H2/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17643
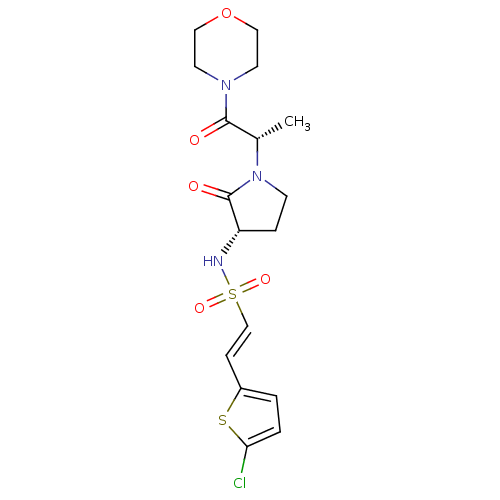
((E)-2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(m...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C17H22ClN3O5S2/c1-12(16(22)20-7-9-26-10-8-20)21-6-4-14(17(21)23)19-28(24,25)11-5-13-2-3-15(18)27-13/h2-3,5,11-12,14,19H,4,6-10H2,1H3/b11-5+/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338687

((R/S)-6-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H24ClN3O3S/c1-26-22-9-4-17-13-19(6-8-21(17)22)28-11-10-23(24(28)29)27-32(30,31)20-7-3-15-12-18(25)5-2-16(15)14-20/h2-3,5-8,12-14,22-23,26-27H,4,9-11H2,1H3/t22?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338679

((E)-2-(5-chlorothiophen-2-yl)-N-((S)-1-(2-fluoro-4...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-19-6-4-14(29-19)8-11-30(27,28)24-17-7-10-25(20(17)26)18-5-3-13(12-15(18)22)16-2-1-9-23-16/h3-6,8,11-12,16-17,23-24H,1-2,7,9-10H2/b11-8+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338685

((R/S)-N-((3S)-1-(1-amino-2,3-dihydro-1H-inden-5-yl...)Show SMILES NC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C23H22ClN3O3S/c24-17-4-1-15-13-19(6-2-14(15)11-17)31(29,30)26-22-9-10-27(23(22)28)18-5-7-20-16(12-18)3-8-21(20)25/h1-2,4-7,11-13,21-22,26H,3,8-10,25H2/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338679

((E)-2-(5-chlorothiophen-2-yl)-N-((S)-1-(2-fluoro-4...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-19-6-4-14(29-19)8-11-30(27,28)24-17-7-10-25(20(17)26)18-5-3-13(12-15(18)22)16-2-1-9-23-16/h3-6,8,11-12,16-17,23-24H,1-2,7,9-10H2/b11-8+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338683

((R/S)-6-chloro-N-((3S)-1-(2-fluoro-4-(1-methylpyrr...)Show SMILES CN1CCCC1c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H25ClFN3O3S/c1-29-11-2-3-23(29)18-6-9-24(21(27)15-18)30-12-10-22(25(30)31)28-34(32,33)20-8-5-16-13-19(26)7-4-17(16)14-20/h4-9,13-15,22-23,28H,2-3,10-12H2,1H3/t22-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338690

(6-CHLORO-N-{(3S)-1-[(1S)-1-(DIMETHYLAMINO)-2,3-DIH...)Show SMILES CN(C)[C@@H]1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-28(2)24-10-5-18-14-20(7-9-22(18)24)29-12-11-23(25(29)30)27-33(31,32)21-8-4-16-13-19(26)6-3-17(16)15-21/h3-4,6-9,13-15,23-24,27H,5,10-12H2,1-2H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338684
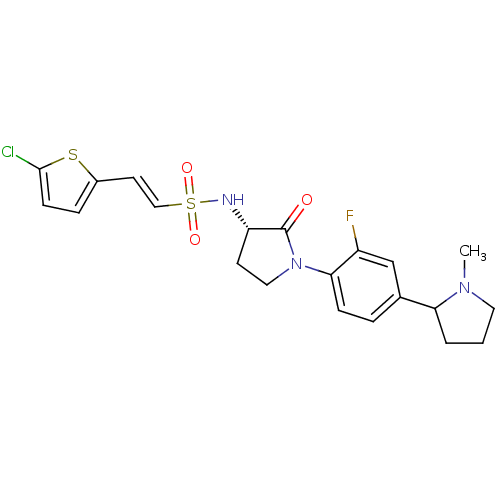
((R/S)-(E)-2-(5-chlorothiophen-2-yl)-N-((3S)-1-(2-f...)Show SMILES CN1CCCC1c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C21H23ClFN3O3S2/c1-25-10-2-3-18(25)14-4-6-19(16(23)13-14)26-11-8-17(21(26)27)24-31(28,29)12-9-15-5-7-20(22)30-15/h4-7,9,12-13,17-18,24H,2-3,8,10-11H2,1H3/b12-9+/t17-,18?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338681

((R/S)-N-((3S)-1-(2-fluoro-4-(pyrrolidin-2-yl)pheny...)Show SMILES Fc1cc(ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc[nH]c3c2)C1=O)C1CCCN1 |r| Show InChI InChI=1S/C22H23FN4O3S/c23-17-12-15(18-2-1-9-24-18)4-6-21(17)27-11-8-19(22(27)28)26-31(29,30)16-5-3-14-7-10-25-20(14)13-16/h3-7,10,12-13,18-19,24-26H,1-2,8-9,11H2/t18?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data