

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University Curated by ChEMBL | Assay Description Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) assessed as N-methyldihydronicotinamide oxidation per mg of protein a... | J Nat Prod 74: 129-36 (2011) Article DOI: 10.1021/np100373f BindingDB Entry DOI: 10.7270/Q20865MS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50339150 (5,7-dihydroxy-2-(4-hydroxyphenyl)-3-((2R,3S,4S,5S,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University Curated by ChEMBL | Assay Description Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) assessed as N-methyldihydronicotinamide oxidation per mg of protein a... | J Nat Prod 74: 129-36 (2011) Article DOI: 10.1021/np100373f BindingDB Entry DOI: 10.7270/Q20865MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339149 (CHEMBL1689259 | beta-(3,4-dihydroxyphenyl)ethyl-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University Curated by ChEMBL | Assay Description Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay | J Nat Prod 74: 129-36 (2011) Article DOI: 10.1021/np100373f BindingDB Entry DOI: 10.7270/Q20865MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339147 (CHEMBL1689257 | beta-(4-hydroxyphenyl)ethyl-4-O-E-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University Curated by ChEMBL | Assay Description Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay | J Nat Prod 74: 129-36 (2011) Article DOI: 10.1021/np100373f BindingDB Entry DOI: 10.7270/Q20865MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339148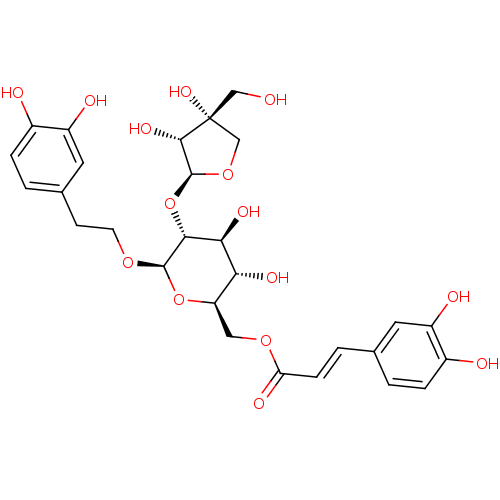 (CHEMBL1689258 | beta-(3,4-dihydroxyphenyl)ethyl-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University Curated by ChEMBL | Assay Description Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay | J Nat Prod 74: 129-36 (2011) Article DOI: 10.1021/np100373f BindingDB Entry DOI: 10.7270/Q20865MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50269516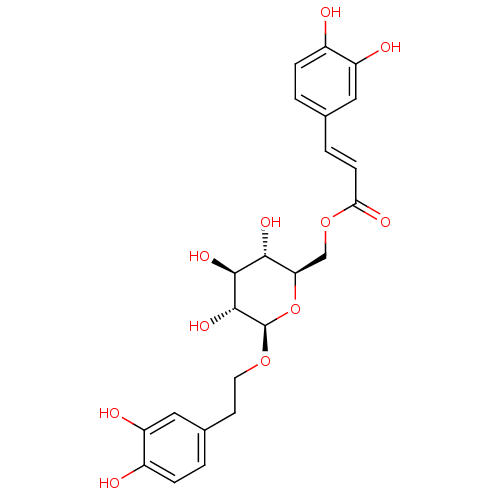 (CHEMBL518414 | Calceolarioside B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University Curated by ChEMBL | Assay Description Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay | J Nat Prod 74: 129-36 (2011) Article DOI: 10.1021/np100373f BindingDB Entry DOI: 10.7270/Q20865MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||