

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341578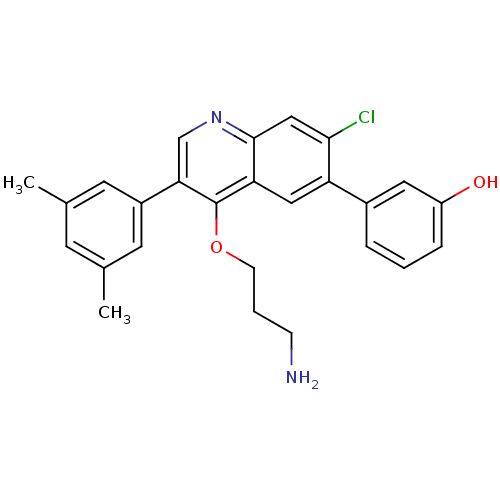 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575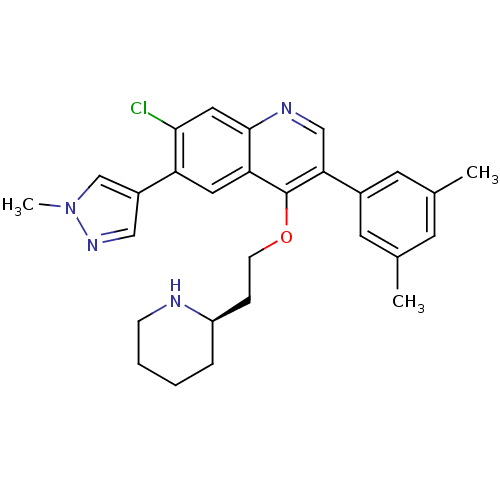 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572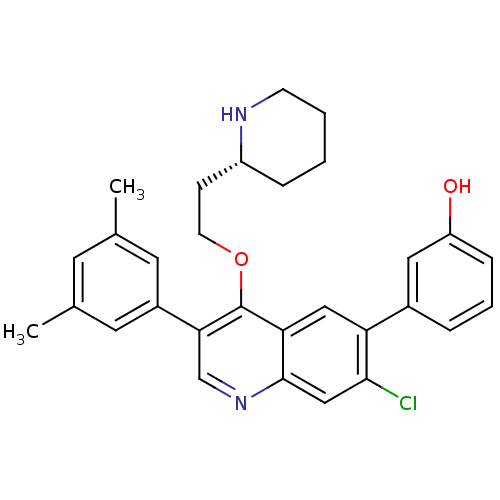 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574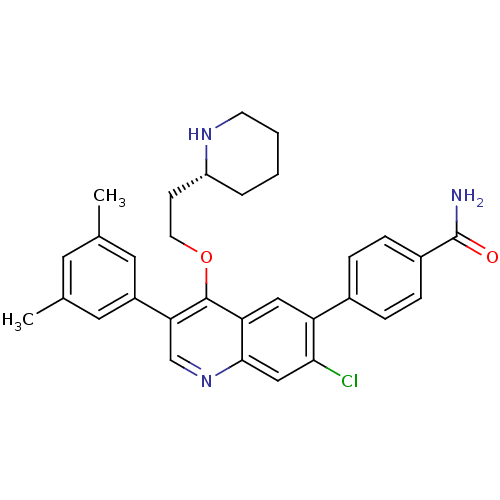 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341562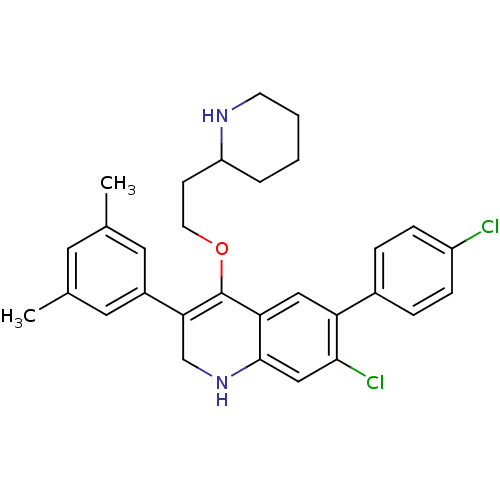 (7-chloro-6-(4-chlorophenyl)-3-(3,5-dimethylphenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341571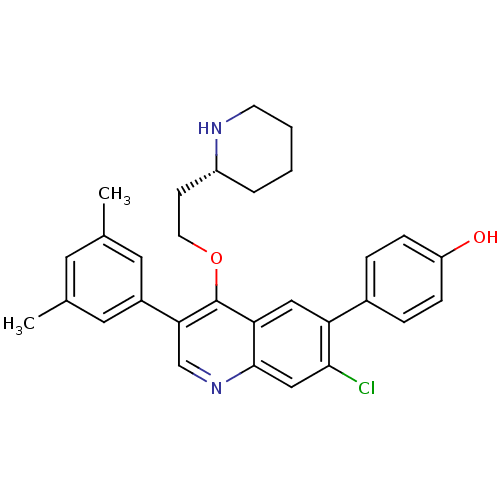 (4-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341577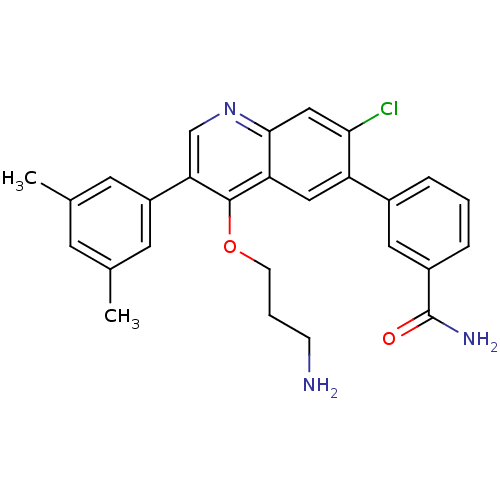 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341573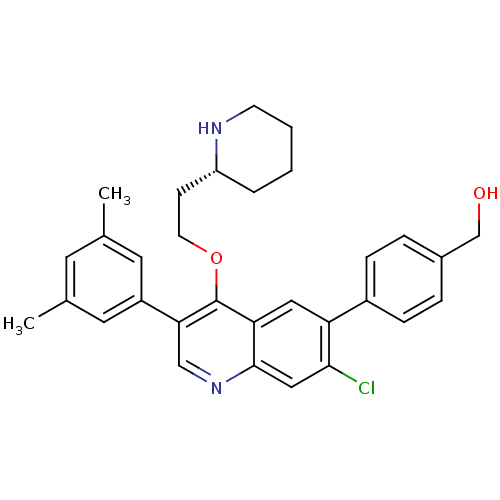 (CHEMBL1766098 | {4-[7-Chloro-3-(3,5-dimethylphenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341570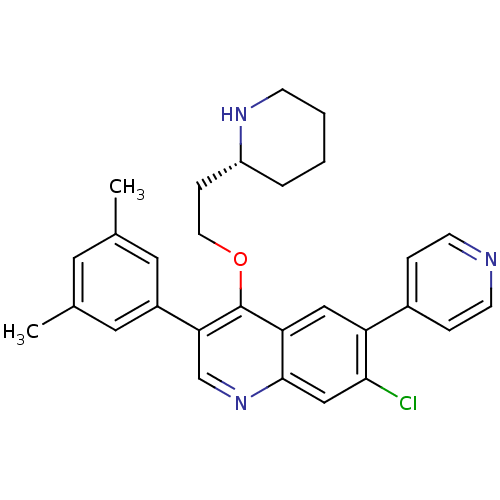 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341569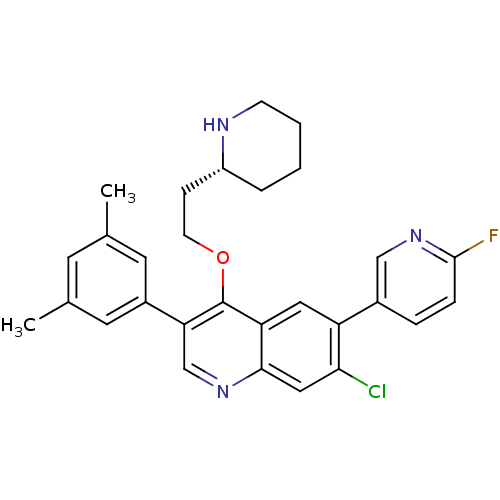 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-fluoropyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341564 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341568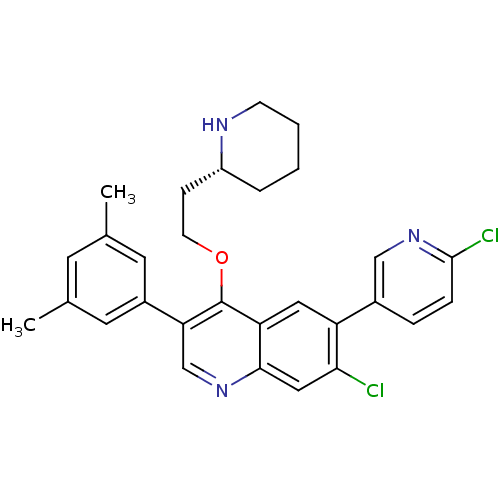 (7-Chloro-6-(6-chloropyridin-3-yl)-3-(3,5-dimethylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341579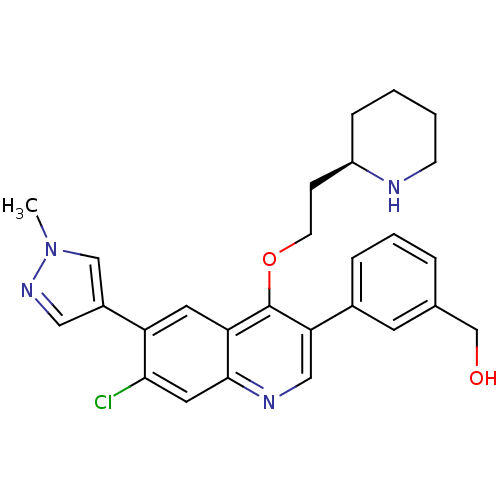 (CHEMBL1766104 | {3-[7-Chloro-6-(1-methyl-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341580 (CHEMBL1766105 | [(4-{2-[(2R)-Piperidin-2-yl]ethoxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341563 (7-chloro-3-(3,5-dimethylphenyl)-6-phenyl-4-(2-(pip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341565 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341566 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341567 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-methoxypyridi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341576 ((S)-7-chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341560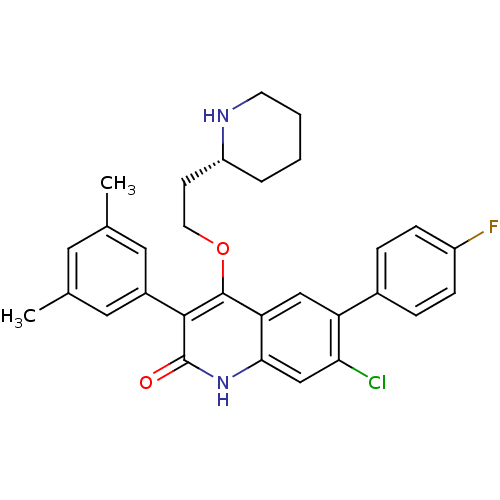 ((R)-7-chloro-3-(3,5-dimethylphenyl)-6-(4-fluorophe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341561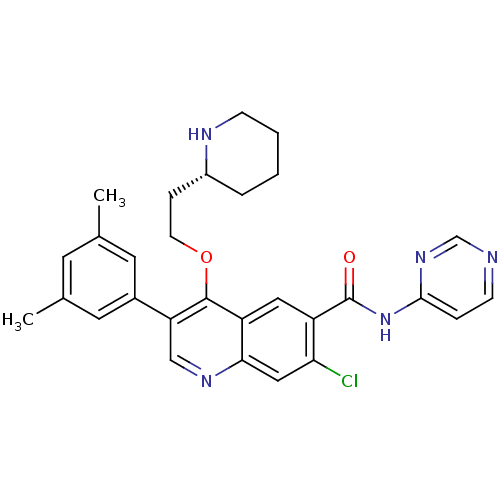 ((R)-7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341559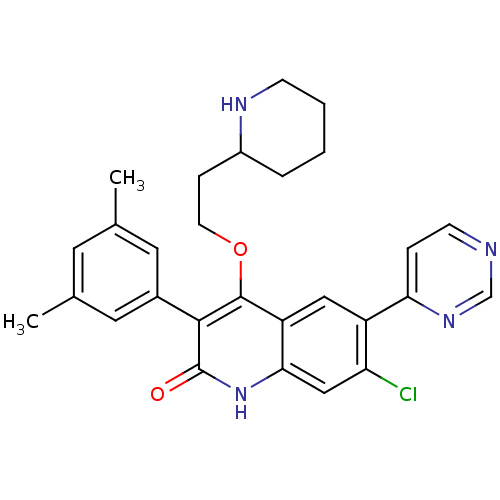 (7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidin-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341565 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341577 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341571 (4-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50341559 (7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidin-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at GnRH receptor by GnRH | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341569 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-fluoropyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341578 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341563 (7-chloro-3-(3,5-dimethylphenyl)-6-phenyl-4-(2-(pip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341579 (CHEMBL1766104 | {3-[7-Chloro-6-(1-methyl-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341564 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341568 (7-Chloro-6-(6-chloropyridin-3-yl)-3-(3,5-dimethylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341580 (CHEMBL1766105 | [(4-{2-[(2R)-Piperidin-2-yl]ethoxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341573 (CHEMBL1766098 | {4-[7-Chloro-3-(3,5-dimethylphenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341570 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341566 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341567 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-methoxypyridi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341576 ((S)-7-chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50341561 ((R)-7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at GnRH receptor by GnRH | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||