

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343722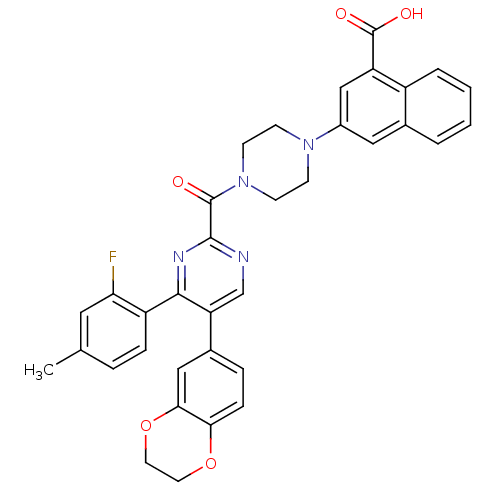 (3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343703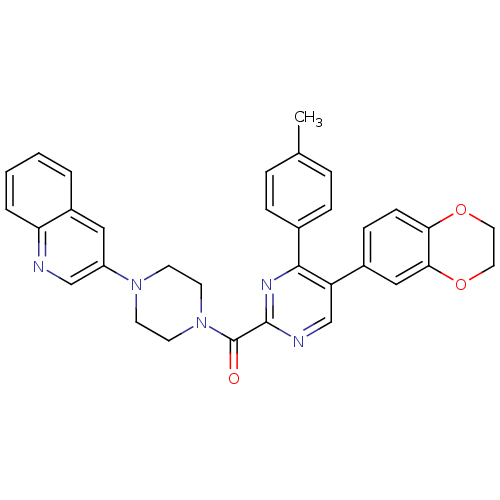 ((5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-p-tolyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343721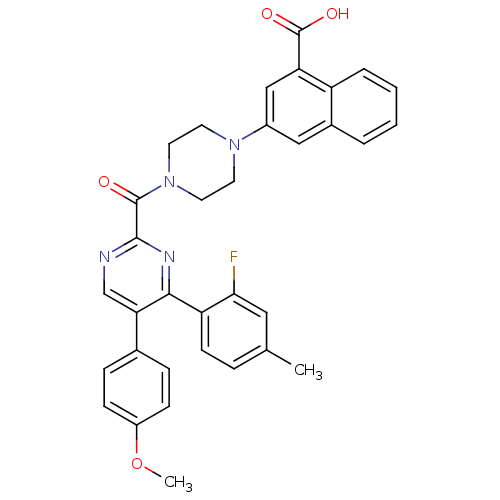 (3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343711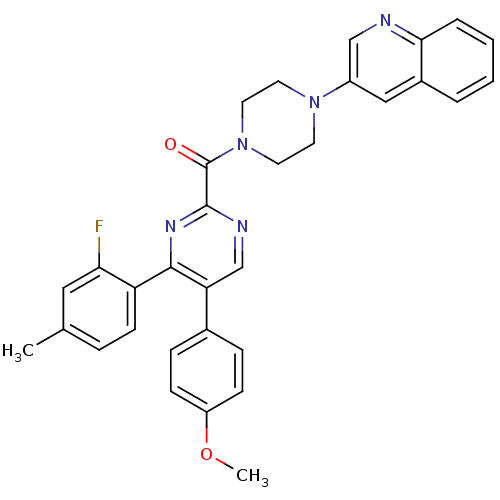 ((4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphenyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343710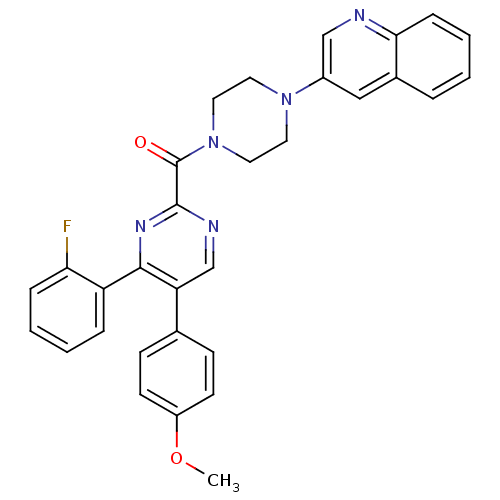 ((4-(2-fluorophenyl)-5-(4-methoxyphenyl)pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343720 (3-(4-(5-(4-methoxyphenyl)-4-p-tolylpyrimidine-2-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343699 ((5-(4-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343700 ((5-(4-ethoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343719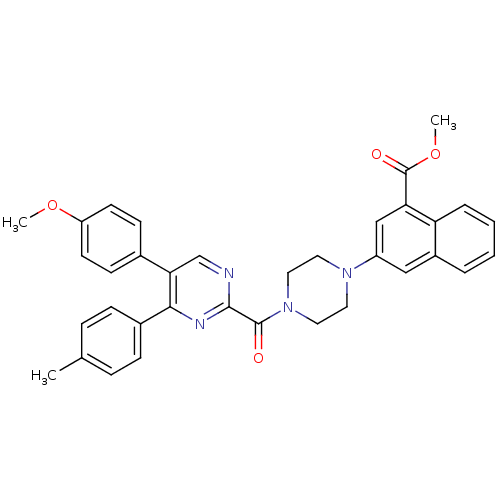 (CHEMBL1774041 | methyl 3-(4-(5-(4-methoxyphenyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343709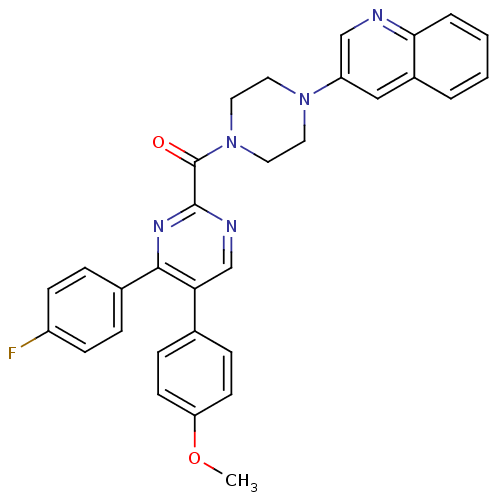 ((4-(4-fluorophenyl)-5-(4-methoxyphenyl)pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343715 ((4-(benzofuran-6-yl)piperazin-1-yl)(5-(4-methoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343713 ((4-(isoquinolin-3-yl)piperazin-1-yl)(5-(4-methoxyp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343712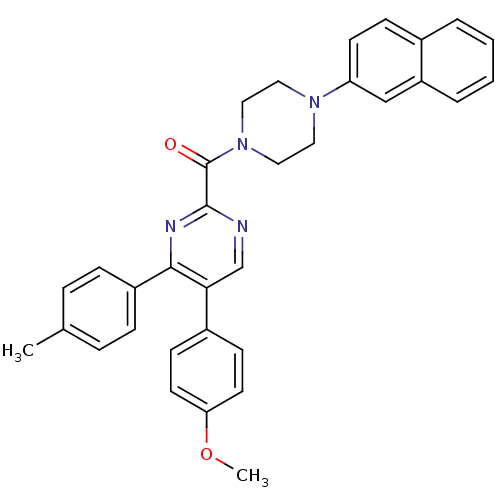 ((5-(4-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343704 ((5-(4-methoxyphenyl)-4-phenylpyrimidin-2-yl)(4-(qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343714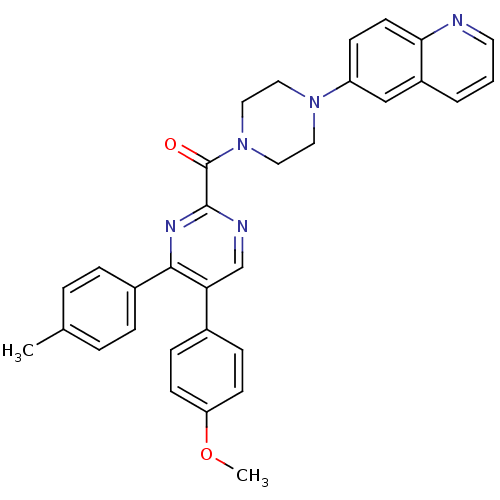 ((5-(4-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343698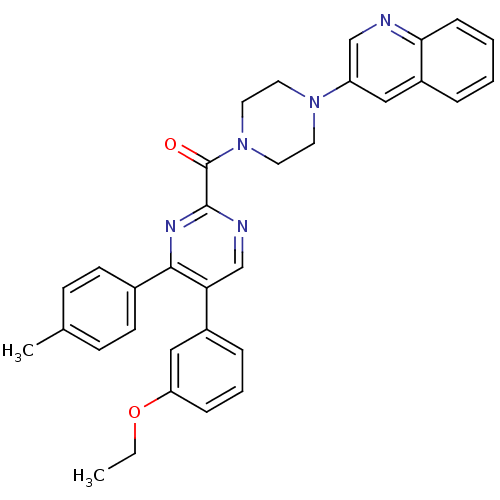 ((5-(3-ethoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343695 ((5-(3-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343707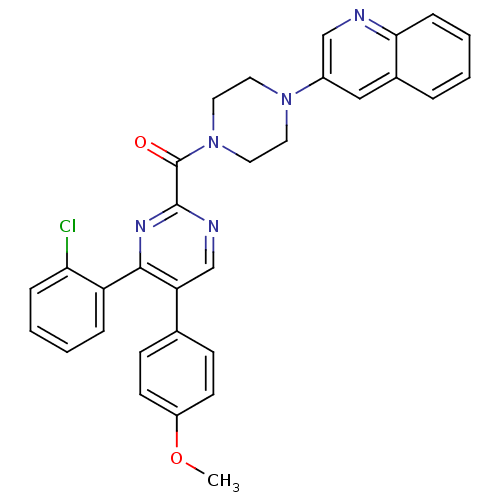 ((4-(2-chlorophenyl)-5-(4-methoxyphenyl)pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343697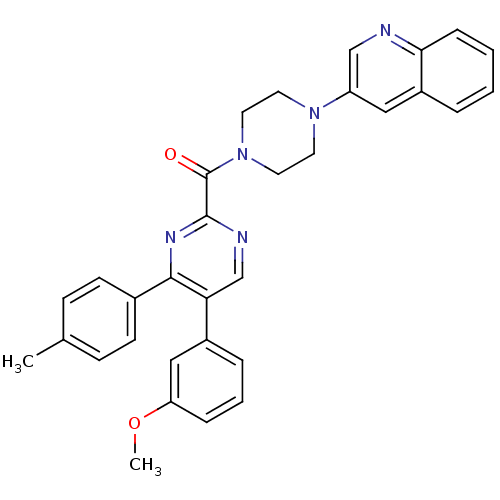 ((5-(3-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343694 ((5-(3-methoxyphenyl)-6-p-tolylpyridin-2-yl)(4-(nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343708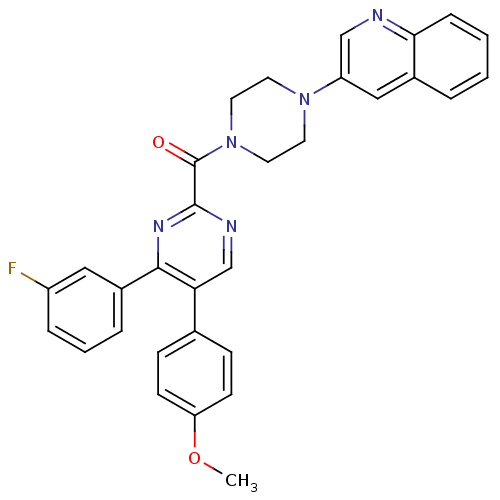 ((4-(3-fluorophenyl)-5-(4-methoxyphenyl)pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343705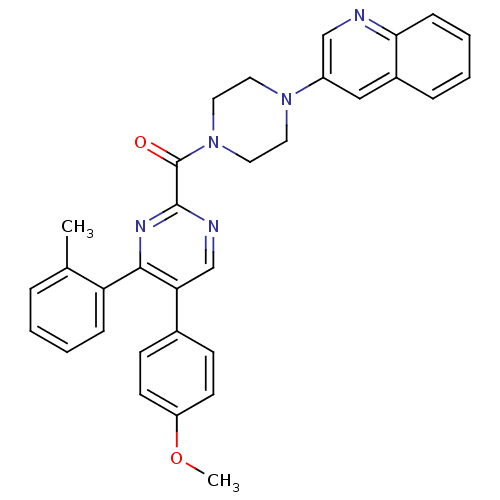 ((5-(4-methoxyphenyl)-4-o-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343716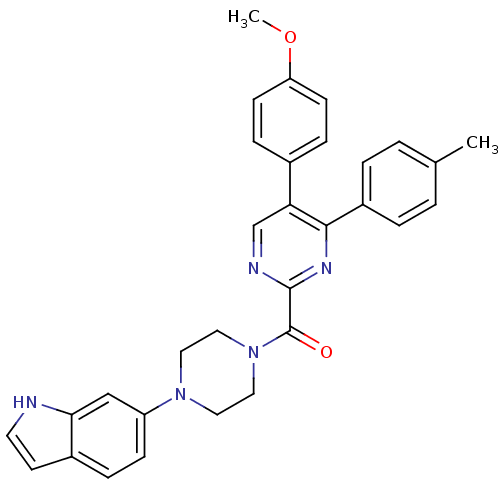 ((4-(1H-indol-6-yl)piperazin-1-yl)(5-(4-methoxyphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343718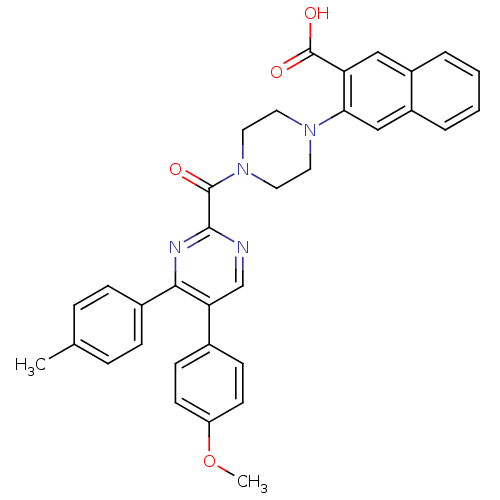 (3-(4-(5-(4-methoxyphenyl)-4-p-tolylpyrimidine-2-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Homo sapiens (Human)) | BDBM50343721 (3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343696 ((5-(2-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343706 ((5-(4-methoxyphenyl)-4-m-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Homo sapiens (Human)) | BDBM50343722 (3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343717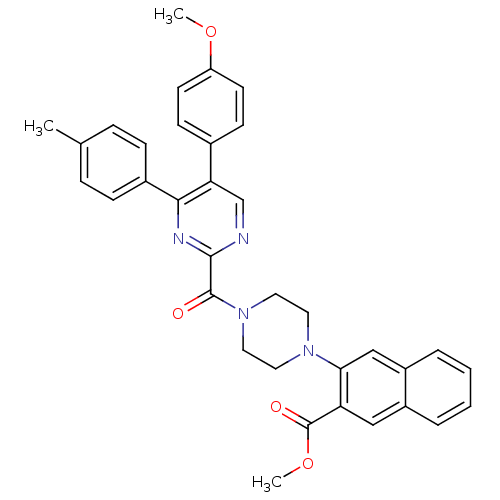 (CHEMBL1774039 | methyl 3-(4-(5-(4-methoxyphenyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 587 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343701 ((5-(3,4-dimethoxyphenyl)-4-p-tolylpyrimidin-2-yl)(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 857 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343702 ((5-(3,4-dihydroxyphenyl)-4-p-tolylpyrimidin-2-yl)(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343692 ((5-(3-methoxyphenyl)-6-p-tolylpyrazin-2-yl)(4-(nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily E member 1 (Homo sapiens (Human)) | BDBM50343721 (3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of IKr | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343691 ((6-(3-methoxyphenyl)-5-p-tolylpyrazin-2-yl)(4-(nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343693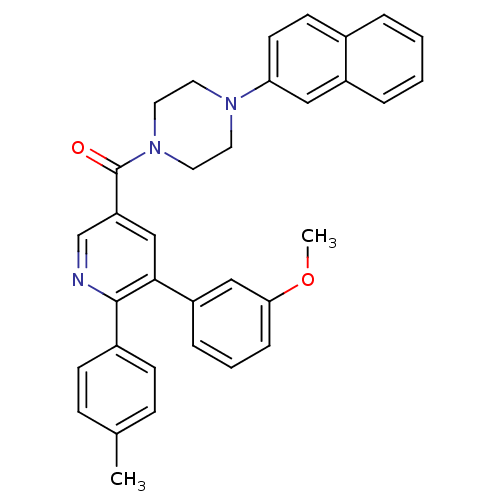 ((5-(3-methoxyphenyl)-6-p-tolylpyridin-3-yl)(4-(nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50343721 (3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of CCK2 receptor | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50343722 (3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of CCK2 receptor | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily E member 1 (Homo sapiens (Human)) | BDBM50343722 (3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of IKr | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343696 ((5-(2-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 286 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50343722 (3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at CB2 receptor | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343698 ((5-(3-ethoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343699 ((5-(4-methoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343700 ((5-(4-ethoxyphenyl)-4-p-tolylpyrimidin-2-yl)(4-(qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343701 ((5-(3,4-dimethoxyphenyl)-4-p-tolylpyrimidin-2-yl)(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 256 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343702 ((5-(3,4-dihydroxyphenyl)-4-p-tolylpyrimidin-2-yl)(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343703 ((5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-p-tolyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343704 ((5-(4-methoxyphenyl)-4-phenylpyrimidin-2-yl)(4-(qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343705 ((5-(4-methoxyphenyl)-4-o-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343706 ((5-(4-methoxyphenyl)-4-m-tolylpyrimidin-2-yl)(4-(q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 196 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50343707 ((4-(2-chlorophenyl)-5-(4-methoxyphenyl)pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... | Bioorg Med Chem Lett 21: 2911-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.069 BindingDB Entry DOI: 10.7270/Q2TB177X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |