

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346205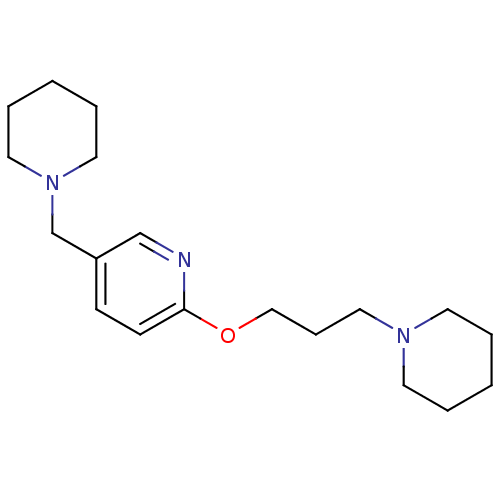 (5-Piperidin-1-ylmethyl-2-(3-piperidin-1-yl-propoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346208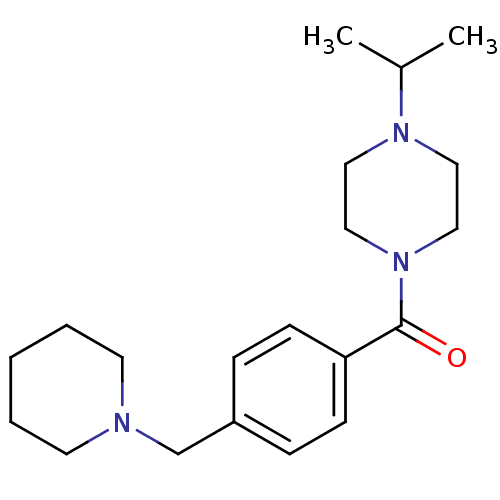 ((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346207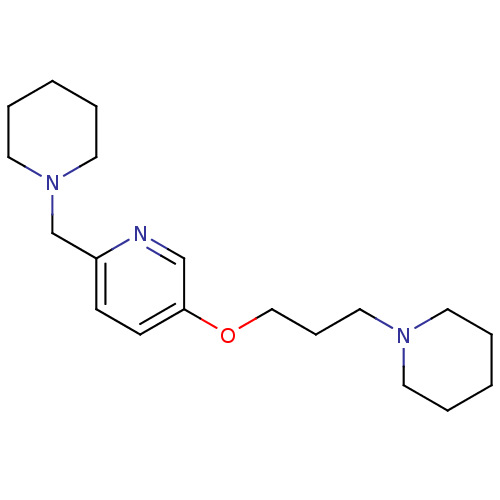 (2-Piperdin-1-ylmethyl-5-(3-piperdin-1-yl-propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50217569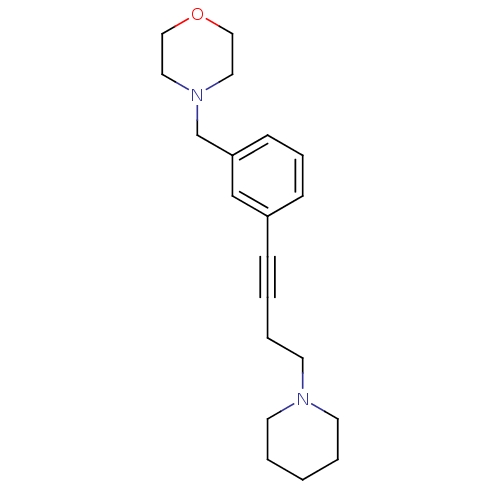 (4-(3-(4-(piperidin-1-yl)but-1-ynyl)benzyl)morpholi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346201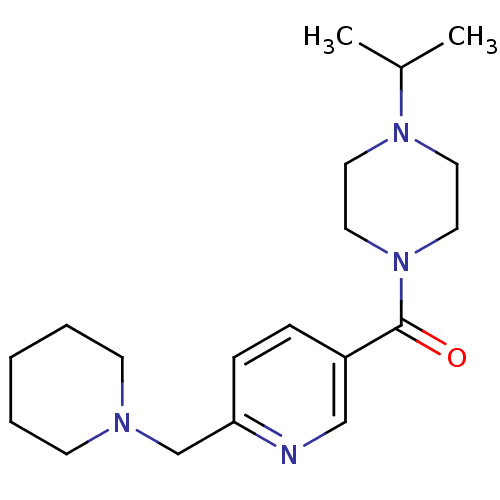 ((4-Isopropyl-piperazin-1-yl)-(6-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346199 ((4-Isopropyl-piperazin-1-yl)-(5-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146835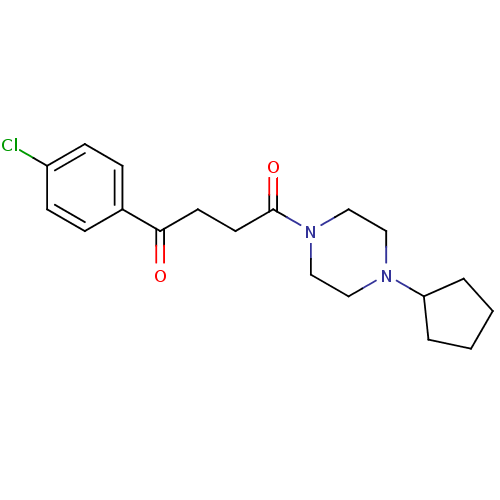 (1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346196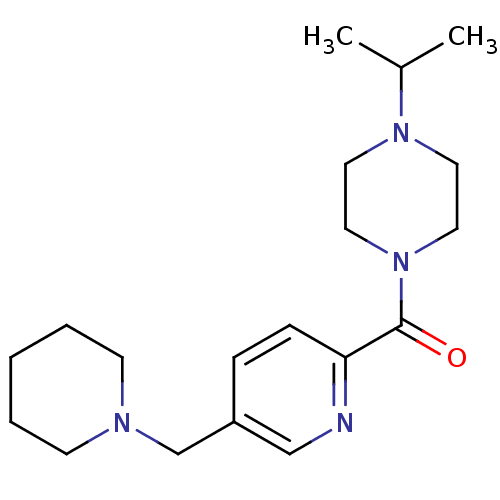 ((4-Isopropyl-piperazin-1-yl)-(5-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50217581 (4-(4-(1-isopropylpiperidin-4-yloxy)benzyl)morpholi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346189 ((4-Isopropyl-piperazin-1-yl)-(5-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346187 ((4-Isopropyl-piperazin-1-yl)-(5-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346204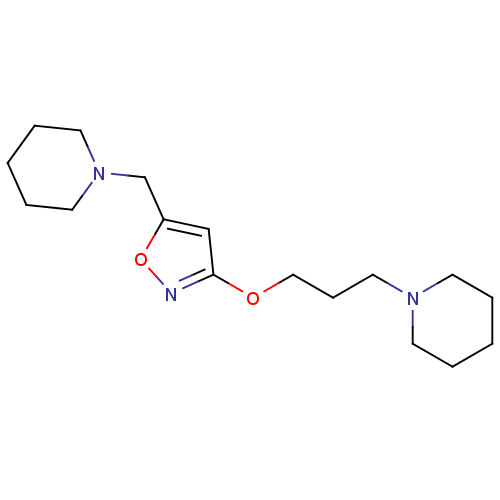 (3-(3-(piperidin-1-yl)propoxy)-5-(piperidin-1-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346182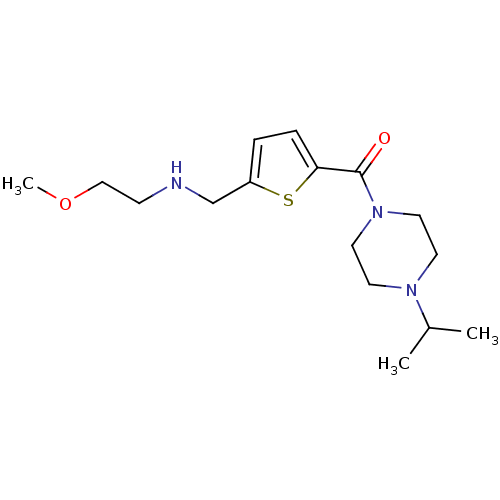 ((4-Isopropyl-piperazin-1-yl)-{5-[(2-methoxy-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346208 ((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat cortical hemispheres | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346197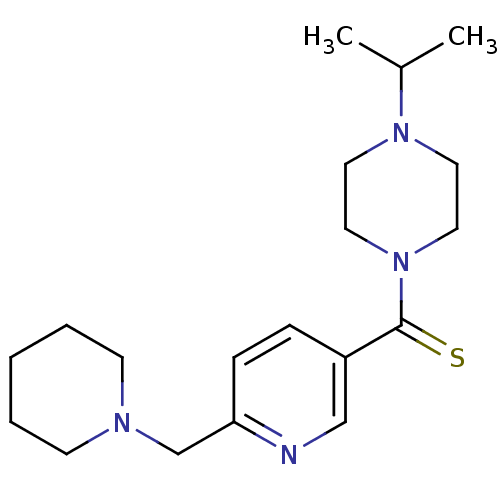 ((4-Isopropyl-piperazin-1-yl)-(6-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346206 (4-[5-(3-Piperidin-1-yl-propoxy)-pyridin-2-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346201 ((4-Isopropyl-piperazin-1-yl)-(6-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat cortical hemispheres | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346184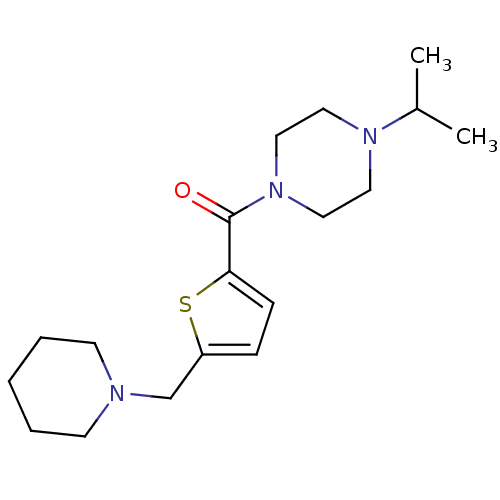 ((4-Isopropyl-piperazin-1-yl)-(5-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346200 ((4-Isopropyl-piperazin-1-yl)-(6-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346198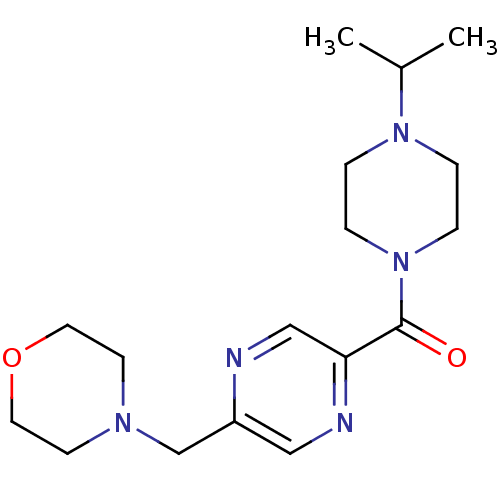 ((4-Isopropyl-piperazin-1-yl)-(5-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346183 ((4-Isopropyl-piperazin-1-yl)-(5-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50177729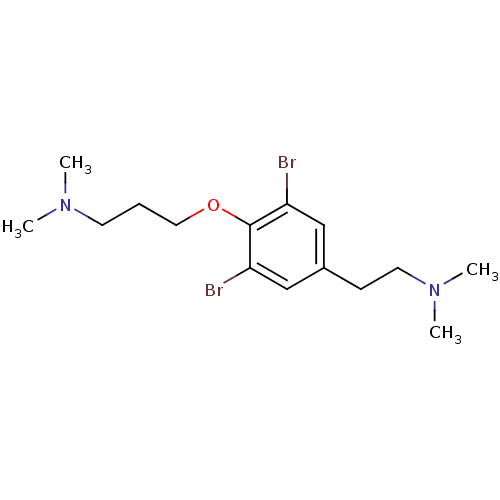 (Aplysamine-1 | CHEMBL380697 | aplysamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346181 ((4-Isopropyl-yl)-(1-methyl-4-piperidin-1-ylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346185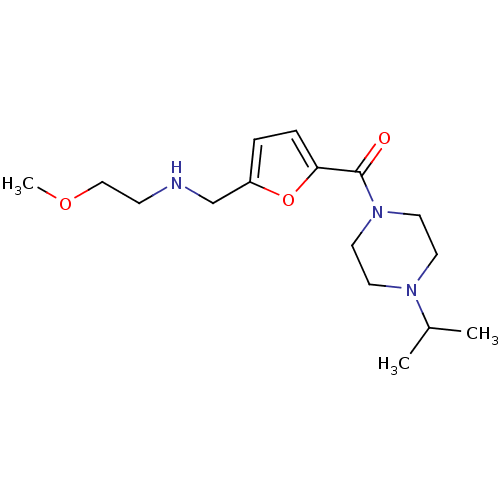 ((4-Isopropyl-piperazin-1-yl)-{5-[(2-methoxy-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346188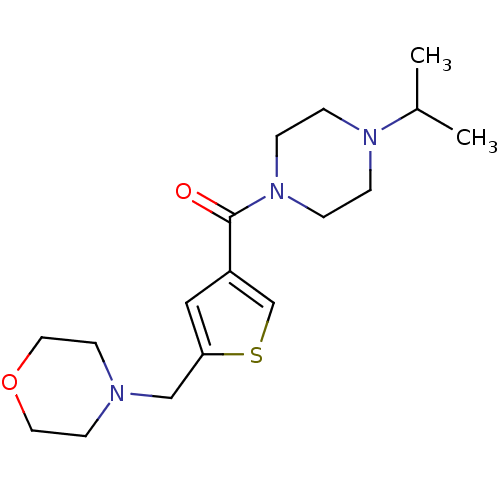 ((4-Isopropyl-piperazin-1-yl)-(5-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346186 ((4-Isopropyl-piperazin-1-yl)-(5-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346202 ((2-Methoxy-ethyl)-[3-(3-piperidin-1-yl-propoxy)-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346203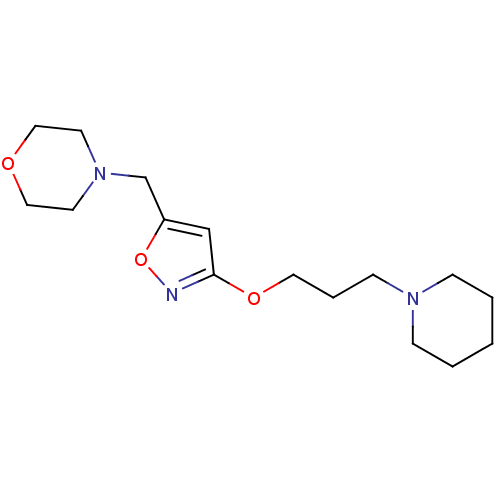 (4-[3-(3-Piperidin-1-yl-propoxy)-isoxazol-5-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346192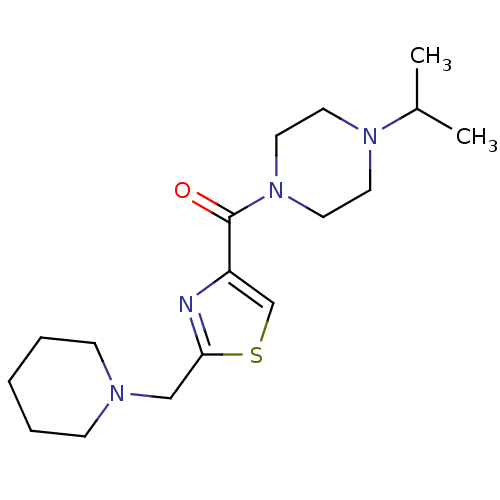 ((4-Isopropyl-piperazin-1-yl)-(2-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346180 ((4-Isopropyl-piperazin-1-yl)-(1-methyl-4-morpholin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346195 ((4-Isopropyl-piperazin-1-yl)-(2-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346190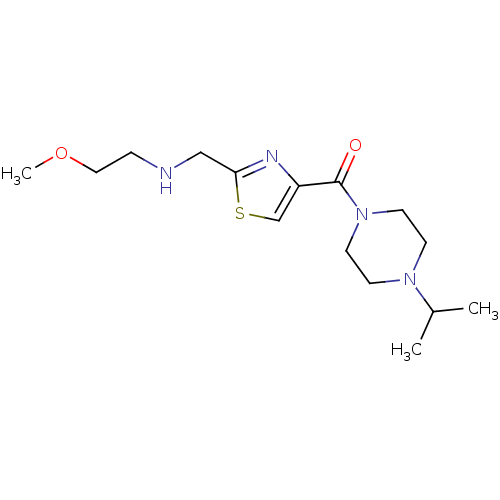 ((4-Isopropyl-piperazin-1-yl)-{2-[(2-methoxy-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346193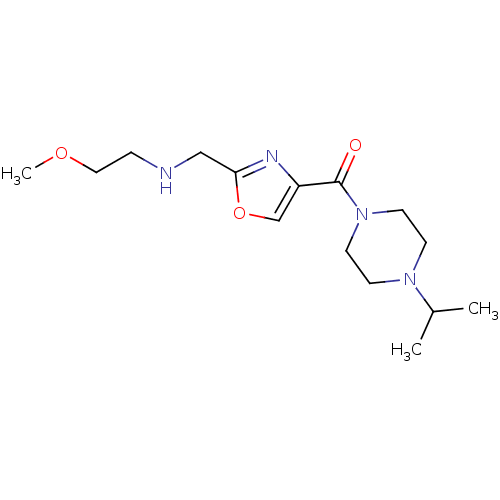 ((4-Isopropyl-piperazin-1-yl)-{2-[(2-methoxy-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346191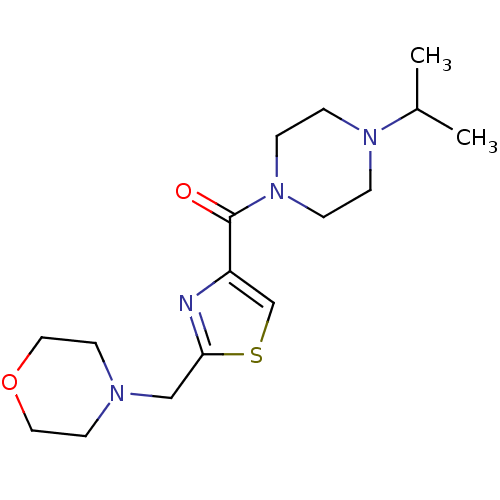 ((4-Isopropyl-piperazin-1-yl)-(2-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 679 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346194 ((4-Isopropyl-piperazin-1-yl)-(2-morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346208 ((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat cortical hemispheres assessed as inhibition of forskolin stimulated cAMP accumulation after 6 hrs | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346201 ((4-Isopropyl-piperazin-1-yl)-(6-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat cortical hemispheres assessed as inhibition of forskolin stimulated cAMP accumulation after 6 hrs | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||