

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354089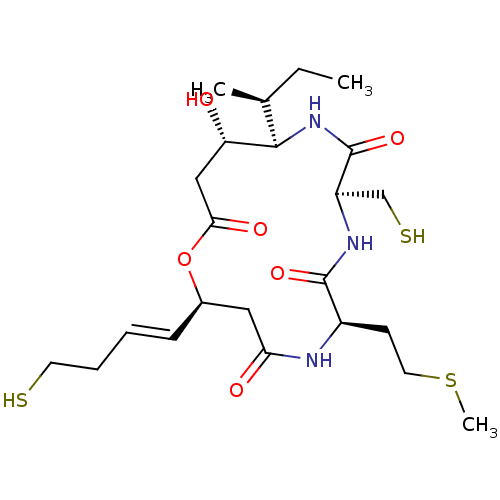 (CHEMBL1836144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354087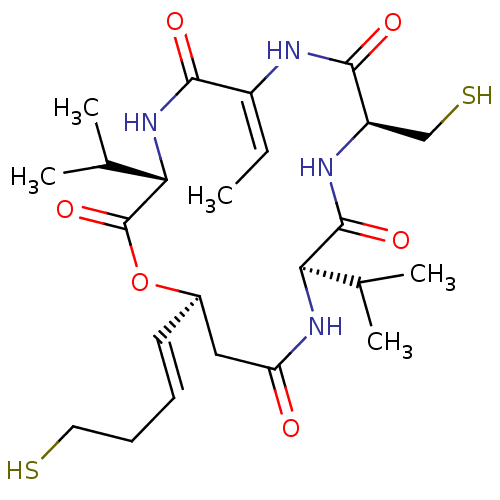 (CHEMBL1836143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354085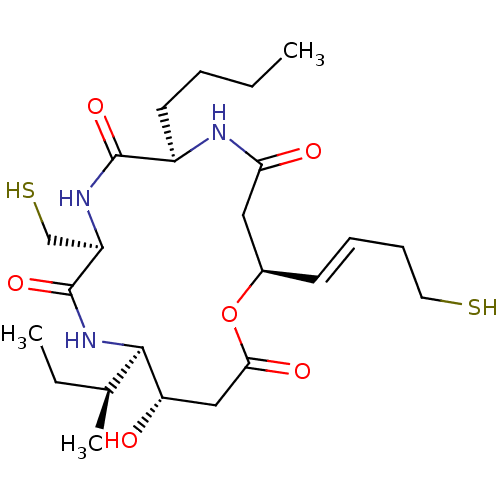 (CHEMBL1836042) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 0... | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354084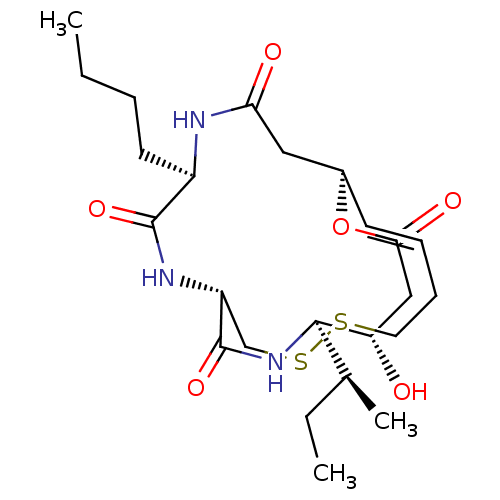 (CHEMBL1836145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354087 (CHEMBL1836143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 0... | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 1... | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354088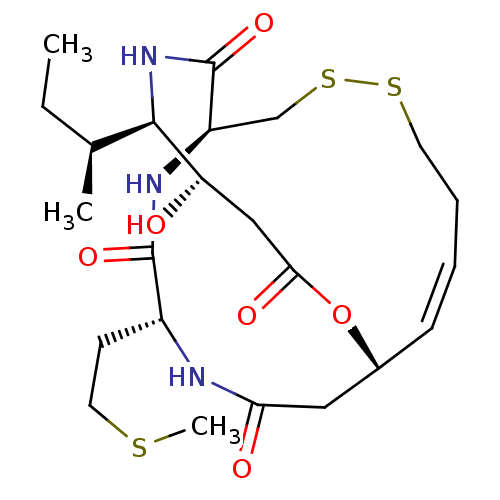 (CHEMBL1836142) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354087 (CHEMBL1836143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354089 (CHEMBL1836144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354087 (CHEMBL1836143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354085 (CHEMBL1836042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354085 (CHEMBL1836042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354089 (CHEMBL1836144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50354087 (CHEMBL1836143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354088 (CHEMBL1836142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50354089 (CHEMBL1836144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354084 (CHEMBL1836145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50354089 (CHEMBL1836144) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50354087 (CHEMBL1836143) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354085 (CHEMBL1836042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354084 (CHEMBL1836145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50354085 (CHEMBL1836042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50354084 (CHEMBL1836145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354084 (CHEMBL1836145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50354085 (CHEMBL1836042) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354089 (CHEMBL1836144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50354084 (CHEMBL1836145) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354088 (CHEMBL1836142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50354088 (CHEMBL1836142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50354088 (CHEMBL1836142) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354088 (CHEMBL1836142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||