 Found 143 hits Enz. Inhib. hit(s) with all data for entry = 50034012
Found 143 hits Enz. Inhib. hit(s) with all data for entry = 50034012 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR1 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR3 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type FGFR1 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa... |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type FGFR2 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa... |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR4 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355394
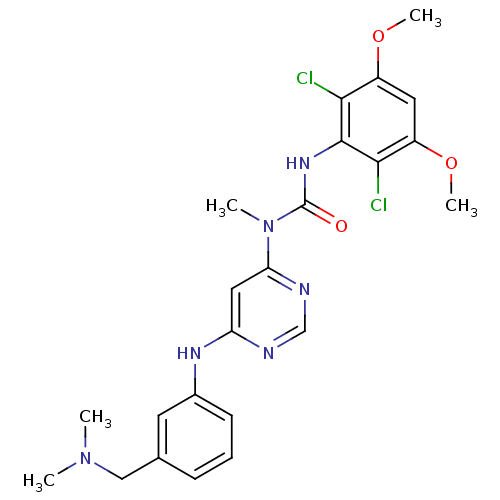
(CHEMBL1834663)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(CN(C)C)c3)ncn2)c1Cl Show InChI InChI=1S/C23H26Cl2N6O3/c1-30(2)12-14-7-6-8-15(9-14)28-18-11-19(27-13-26-18)31(3)23(32)29-22-20(24)16(33-4)10-17(34-5)21(22)25/h6-11,13H,12H2,1-5H3,(H,29,32)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using DBF as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type FGFR4 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa... |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50355388
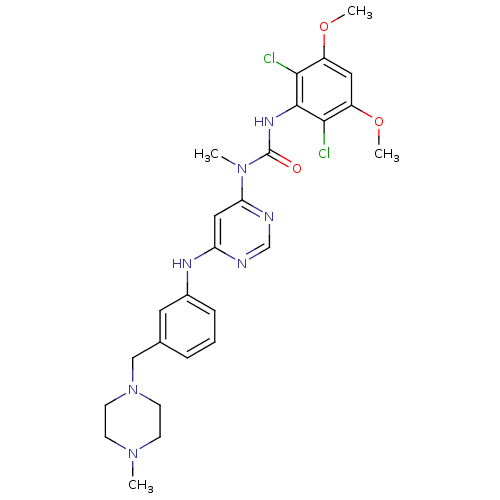
(CHEMBL1834662)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(CN4CCN(C)CC4)c3)ncn2)c1Cl Show InChI InChI=1S/C26H31Cl2N7O3/c1-33-8-10-35(11-9-33)15-17-6-5-7-18(12-17)31-21-14-22(30-16-29-21)34(2)26(36)32-25-23(27)19(37-3)13-20(38-4)24(25)28/h5-7,12-14,16H,8-11,15H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 using CEC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LYN kinase |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355388

(CHEMBL1834662)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(CN4CCN(C)CC4)c3)ncn2)c1Cl Show InChI InChI=1S/C26H31Cl2N7O3/c1-33-8-10-35(11-9-33)15-17-6-5-7-18(12-17)31-21-14-22(30-16-29-21)34(2)26(36)32-25-23(27)19(37-3)13-20(38-4)24(25)28/h5-7,12-14,16H,8-11,15H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using BFC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50234144
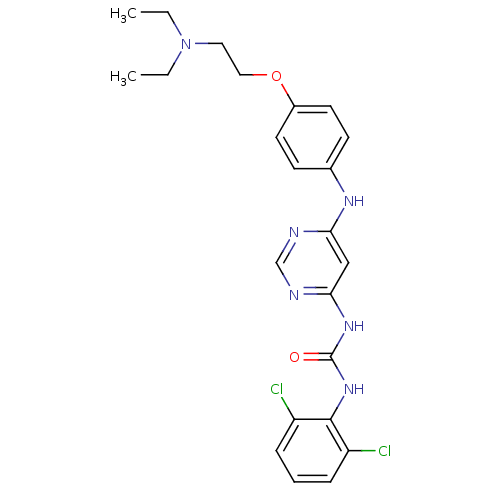
(1-(2,6-dichlorophenyl)-3-(6-(4-(2-(diethylamino)et...)Show SMILES CCN(CC)CCOc1ccc(Nc2cc(NC(=O)Nc3c(Cl)cccc3Cl)ncn2)cc1 Show InChI InChI=1S/C23H26Cl2N6O2/c1-3-31(4-2)12-13-33-17-10-8-16(9-11-17)28-20-14-21(27-15-26-20)29-23(32)30-22-18(24)6-5-7-19(22)25/h5-11,14-15H,3-4,12-13H2,1-2H3,(H3,26,27,28,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant KIT kinase |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50355392
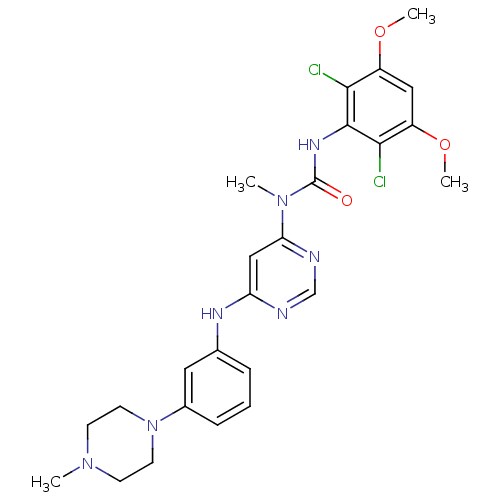
(CHEMBL1834658)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(c3)N3CCN(C)CC3)ncn2)c1Cl Show InChI InChI=1S/C25H29Cl2N7O3/c1-32-8-10-34(11-9-32)17-7-5-6-16(12-17)30-20-14-21(29-15-28-20)33(2)25(35)31-24-22(26)18(36-3)13-19(37-4)23(24)27/h5-7,12-15H,8-11H2,1-4H3,(H,31,35)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 using CEC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 938 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355390
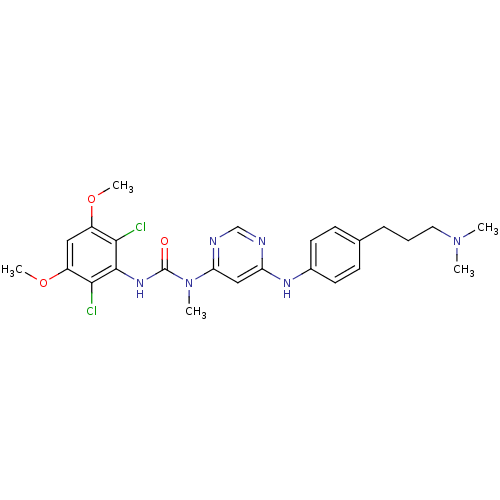
(CHEMBL1834660)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3ccc(CCCN(C)C)cc3)ncn2)c1Cl Show InChI InChI=1S/C25H30Cl2N6O3/c1-32(2)12-6-7-16-8-10-17(11-9-16)30-20-14-21(29-15-28-20)33(3)25(34)31-24-22(26)18(35-4)13-19(36-5)23(24)27/h8-11,13-15H,6-7,12H2,1-5H3,(H,31,34)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using BFC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355392

(CHEMBL1834658)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(c3)N3CCN(C)CC3)ncn2)c1Cl Show InChI InChI=1S/C25H29Cl2N7O3/c1-32-8-10-34(11-9-32)17-7-5-6-16(12-17)30-20-14-21(29-15-28-20)33(2)25(35)31-24-22(26)18(36-3)13-19(37-4)23(24)27/h5-7,12-15H,8-11H2,1-4H3,(H,31,35)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using BFC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50355389
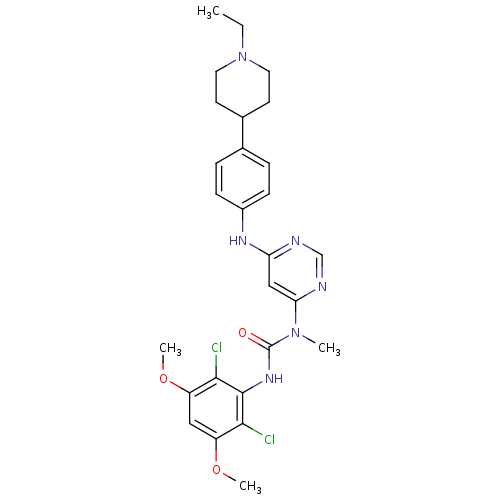
(CHEMBL1834661)Show SMILES CCN1CCC(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C27H32Cl2N6O3/c1-5-35-12-10-18(11-13-35)17-6-8-19(9-7-17)32-22-15-23(31-16-30-22)34(2)27(36)33-26-24(28)20(37-3)14-21(38-4)25(26)29/h6-9,14-16,18H,5,10-13H2,1-4H3,(H,33,36)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 using CEC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant YES kinase |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355389

(CHEMBL1834661)Show SMILES CCN1CCC(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C27H32Cl2N6O3/c1-5-35-12-10-18(11-13-35)17-6-8-19(9-7-17)32-22-15-23(31-16-30-22)34(2)27(36)33-26-24(28)20(37-3)14-21(38-4)25(26)29/h6-9,14-16,18H,5,10-13H2,1-4H3,(H,33,36)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using BFC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50355394

(CHEMBL1834663)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(CN(C)C)c3)ncn2)c1Cl Show InChI InChI=1S/C23H26Cl2N6O3/c1-30(2)12-14-7-6-8-15(9-14)28-18-11-19(27-13-26-18)31(3)23(32)29-22-20(24)16(33-4)10-17(34-5)21(22)25/h6-11,13H,12H2,1-5H3,(H,29,32)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 using CEC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50355392

(CHEMBL1834658)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(c3)N3CCN(C)CC3)ncn2)c1Cl Show InChI InChI=1S/C25H29Cl2N7O3/c1-32-8-10-34(11-9-32)17-7-5-6-16(12-17)30-20-14-21(29-15-28-20)33(2)25(35)31-24-22(26)18(36-3)13-19(37-4)23(24)27/h5-7,12-15H,8-11H2,1-4H3,(H,31,35)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using MFC as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FYN kinase |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of TIE2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ABL kinase |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LCK kinase |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355394

(CHEMBL1834663)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3cccc(CN(C)C)c3)ncn2)c1Cl Show InChI InChI=1S/C23H26Cl2N6O3/c1-30(2)12-14-7-6-8-15(9-14)28-18-11-19(27-13-26-18)31(3)23(32)29-22-20(24)16(33-4)10-17(34-5)21(22)25/h6-11,13H,12H2,1-5H3,(H,29,32)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using DBF as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355391
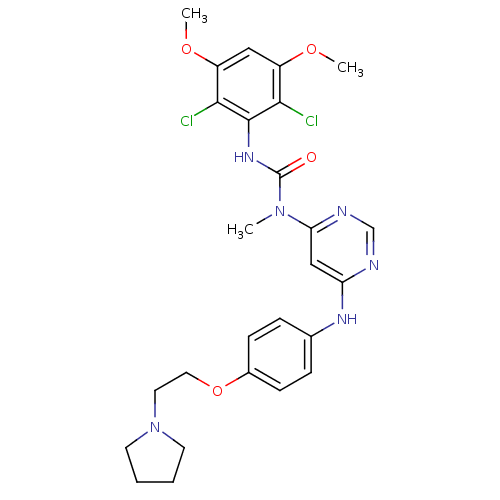
(CHEMBL1834659)Show SMILES COc1cc(OC)c(Cl)c(NC(=O)N(C)c2cc(Nc3ccc(OCCN4CCCC4)cc3)ncn2)c1Cl Show InChI InChI=1S/C26H30Cl2N6O4/c1-33(26(35)32-25-23(27)19(36-2)14-20(37-3)24(25)28)22-15-21(29-16-30-22)31-17-6-8-18(9-7-17)38-13-12-34-10-4-5-11-34/h6-9,14-16H,4-5,10-13H2,1-3H3,(H,32,35)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using DBF as substrate |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KIT juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JAK2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RON-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MET-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LYN-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EPHB1-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ALK juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of TRKB juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLT3-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ROS-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of TYK2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant RET |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data