 Found 92 hits Enz. Inhib. hit(s) with all data for entry = 50049052
Found 92 hits Enz. Inhib. hit(s) with all data for entry = 50049052 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233597
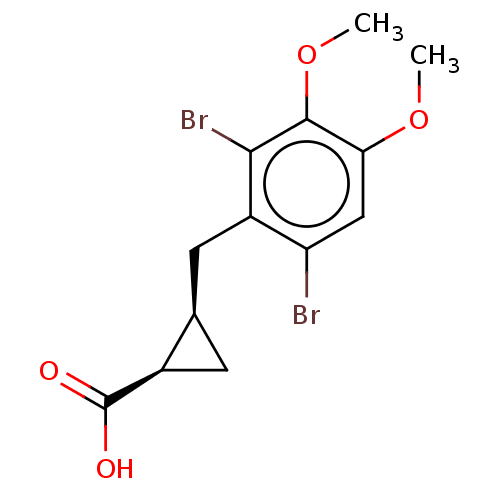
(CHEMBL4091363)Show SMILES COc1cc(Br)c(C[C@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-10-5-9(14)8(11(15)12(10)19-2)4-6-3-7(6)13(16)17/h5-7H,3-4H2,1-2H3,(H,16,17)/t6-,7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233548
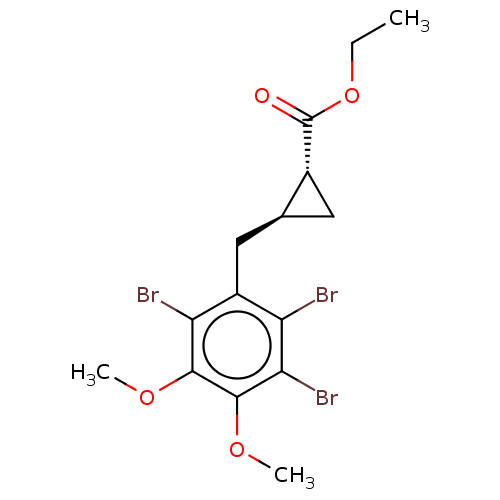
(CHEMBL4064334)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1c(Br)c(Br)c(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H17Br3O4/c1-4-22-15(19)8-5-7(8)6-9-10(16)12(18)14(21-3)13(20-2)11(9)17/h7-8H,4-6H2,1-3H3/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233535
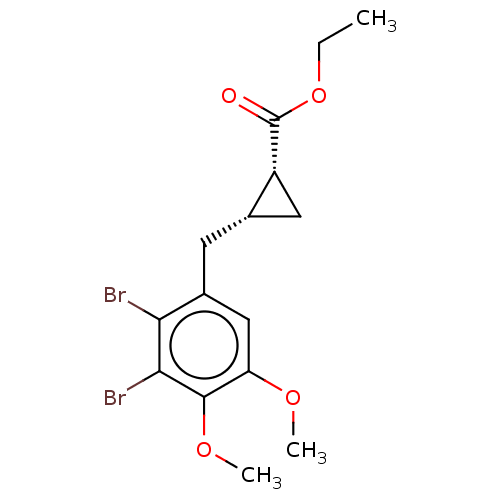
(CHEMBL4086787)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1cc(OC)c(OC)c(Br)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)10-6-8(10)5-9-7-11(19-2)14(20-3)13(17)12(9)16/h7-8,10H,4-6H2,1-3H3/t8-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233544
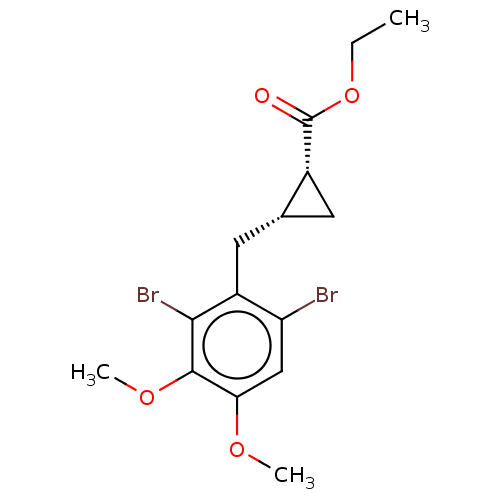
(CHEMBL4065358)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1c(Br)cc(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)9-5-8(9)6-10-11(16)7-12(19-2)14(20-3)13(10)17/h7-9H,4-6H2,1-3H3/t8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233542
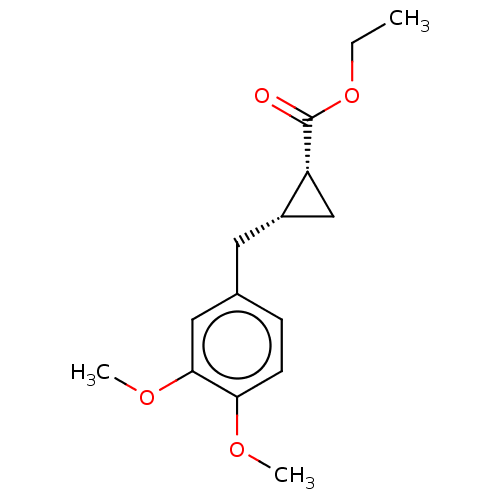
(CHEMBL4070958)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C15H20O4/c1-4-19-15(16)12-9-11(12)7-10-5-6-13(17-2)14(8-10)18-3/h5-6,8,11-12H,4,7,9H2,1-3H3/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233537
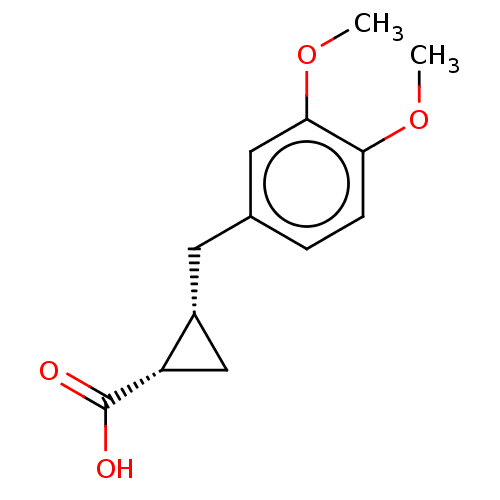
(CHEMBL4062330)Show InChI InChI=1S/C13H16O4/c1-16-11-4-3-8(6-12(11)17-2)5-9-7-10(9)13(14)15/h3-4,6,9-10H,5,7H2,1-2H3,(H,14,15)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233541
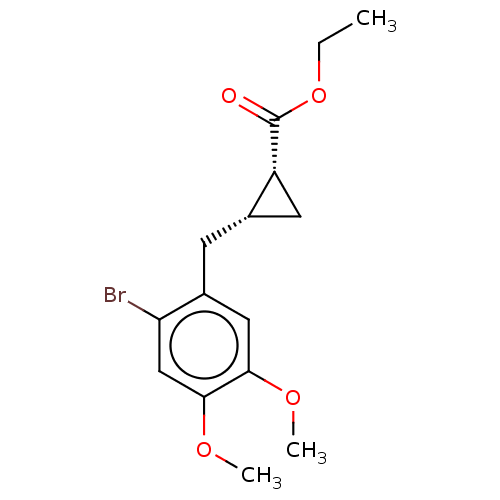
(CHEMBL4103210)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1cc(OC)c(OC)cc1Br |r| Show InChI InChI=1S/C15H19BrO4/c1-4-20-15(17)11-6-9(11)5-10-7-13(18-2)14(19-3)8-12(10)16/h7-9,11H,4-6H2,1-3H3/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233594
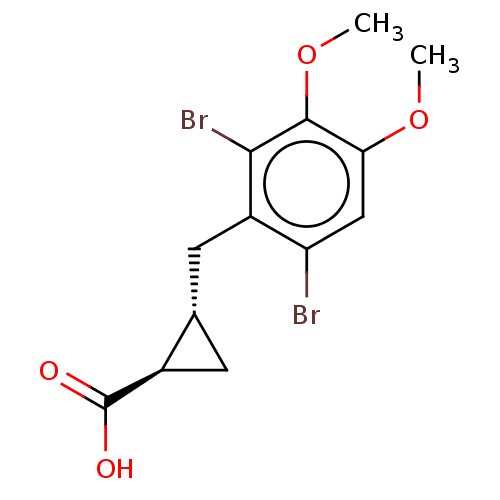
(CHEMBL4060739)Show SMILES COc1cc(Br)c(C[C@@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-10-5-9(14)8(11(15)12(10)19-2)4-6-3-7(6)13(16)17/h5-7H,3-4H2,1-2H3,(H,16,17)/t6-,7+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233535

(CHEMBL4086787)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1cc(OC)c(OC)c(Br)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)10-6-8(10)5-9-7-11(19-2)14(20-3)13(17)12(9)16/h7-8,10H,4-6H2,1-3H3/t8-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233596
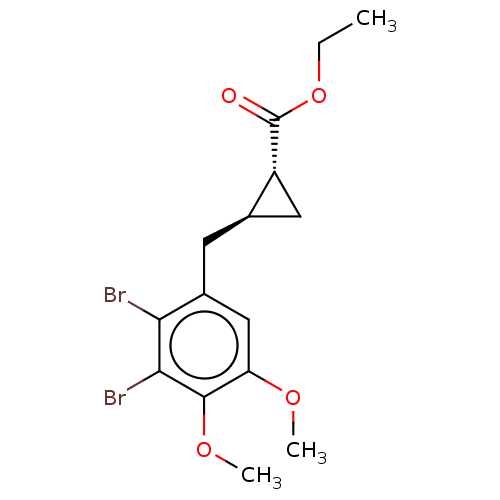
(CHEMBL4068066)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1cc(OC)c(OC)c(Br)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)10-6-8(10)5-9-7-11(19-2)14(20-3)13(17)12(9)16/h7-8,10H,4-6H2,1-3H3/t8-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233536
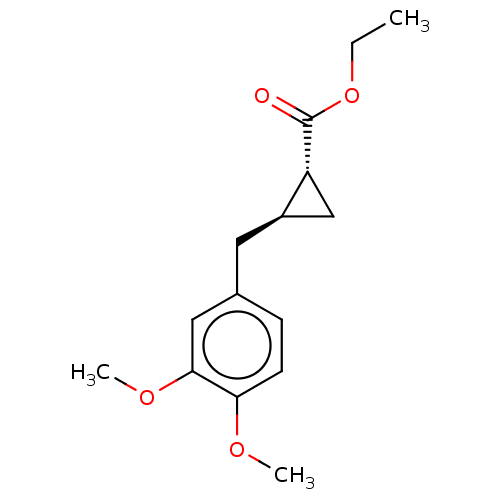
(CHEMBL4090334)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C15H20O4/c1-4-19-15(16)12-9-11(12)7-10-5-6-13(17-2)14(8-10)18-3/h5-6,8,11-12H,4,7,9H2,1-3H3/t11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233543
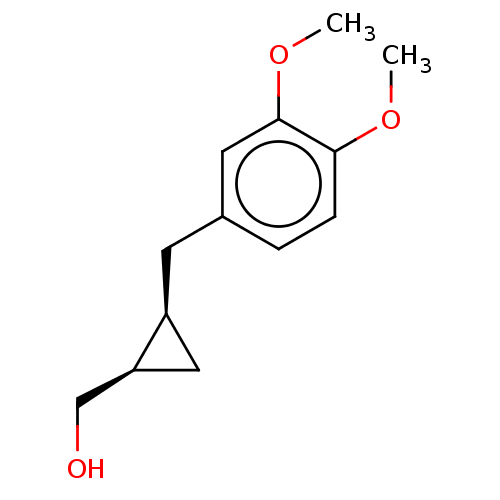
(CHEMBL4089410)Show InChI InChI=1S/C13H18O3/c1-15-12-4-3-9(6-13(12)16-2)5-10-7-11(10)8-14/h3-4,6,10-11,14H,5,7-8H2,1-2H3/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233549

(CHEMBL4092055)Show InChI InChI=1S/C13H15BrO4/c1-17-11-5-8(10(14)6-12(11)18-2)3-7-4-9(7)13(15)16/h5-7,9H,3-4H2,1-2H3,(H,15,16)/t7-,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233593

(CHEMBL4069416)Show SMILES COc1c(Br)c(Br)c(C[C@@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H13Br3O4/c1-19-11-9(15)7(4-5-3-6(5)13(17)18)8(14)10(16)12(11)20-2/h5-6H,3-4H2,1-2H3,(H,17,18)/t5-,6+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233547
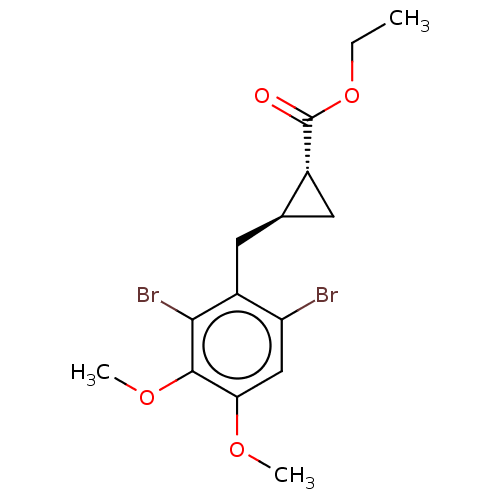
(CHEMBL4095455)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1c(Br)cc(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)9-5-8(9)6-10-11(16)7-12(19-2)14(20-3)13(10)17/h7-9H,4-6H2,1-3H3/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233545

(CHEMBL4105642)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1c(Br)c(Br)c(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H17Br3O4/c1-4-22-15(19)8-5-7(8)6-9-10(16)12(18)14(21-3)13(20-2)11(9)17/h7-8H,4-6H2,1-3H3/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233595
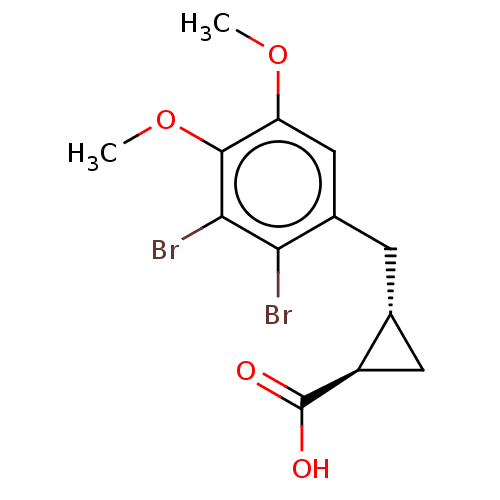
(CHEMBL4082617)Show SMILES COc1cc(C[C@@H]2C[C@H]2C(O)=O)c(Br)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-9-5-7(3-6-4-8(6)13(16)17)10(14)11(15)12(9)19-2/h5-6,8H,3-4H2,1-2H3,(H,16,17)/t6-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233551
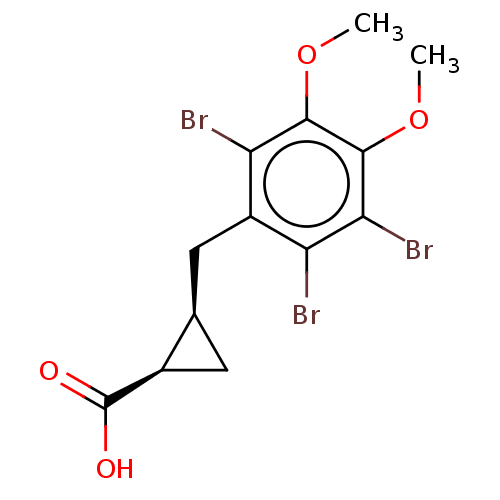
(CHEMBL4099098)Show SMILES COc1c(Br)c(Br)c(C[C@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H13Br3O4/c1-19-11-9(15)7(4-5-3-6(5)13(17)18)8(14)10(16)12(11)20-2/h5-6H,3-4H2,1-2H3,(H,17,18)/t5-,6-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233550

(CHEMBL4063516)Show SMILES COc1cc(C[C@H]2C[C@H]2C(O)=O)c(Br)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-9-5-7(3-6-4-8(6)13(16)17)10(14)11(15)12(9)19-2/h5-6,8H,3-4H2,1-2H3,(H,16,17)/t6-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233546

(CHEMBL4087756)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1cc(OC)c(OC)cc1Br |r| Show InChI InChI=1S/C15H19BrO4/c1-4-20-15(17)11-6-9(11)5-10-7-13(18-2)14(19-3)8-12(10)16/h7-9,11H,4-6H2,1-3H3/t9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233596

(CHEMBL4068066)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1cc(OC)c(OC)c(Br)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)10-6-8(10)5-9-7-11(19-2)14(20-3)13(17)12(9)16/h7-8,10H,4-6H2,1-3H3/t8-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233547

(CHEMBL4095455)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1c(Br)cc(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)9-5-8(9)6-10-11(16)7-12(19-2)14(20-3)13(10)17/h7-9H,4-6H2,1-3H3/t8-,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233594

(CHEMBL4060739)Show SMILES COc1cc(Br)c(C[C@@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-10-5-9(14)8(11(15)12(10)19-2)4-6-3-7(6)13(16)17/h5-7H,3-4H2,1-2H3,(H,16,17)/t6-,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233539
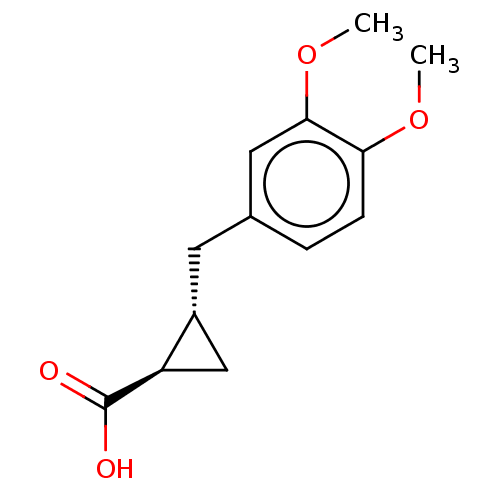
(CHEMBL4068228)Show InChI InChI=1S/C13H16O4/c1-16-11-4-3-8(6-12(11)17-2)5-9-7-10(9)13(14)15/h3-4,6,9-10H,5,7H2,1-2H3,(H,14,15)/t9-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233551

(CHEMBL4099098)Show SMILES COc1c(Br)c(Br)c(C[C@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H13Br3O4/c1-19-11-9(15)7(4-5-3-6(5)13(17)18)8(14)10(16)12(11)20-2/h5-6H,3-4H2,1-2H3,(H,17,18)/t5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233537

(CHEMBL4062330)Show InChI InChI=1S/C13H16O4/c1-16-11-4-3-8(6-12(11)17-2)5-9-7-10(9)13(14)15/h3-4,6,9-10H,5,7H2,1-2H3,(H,14,15)/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233598
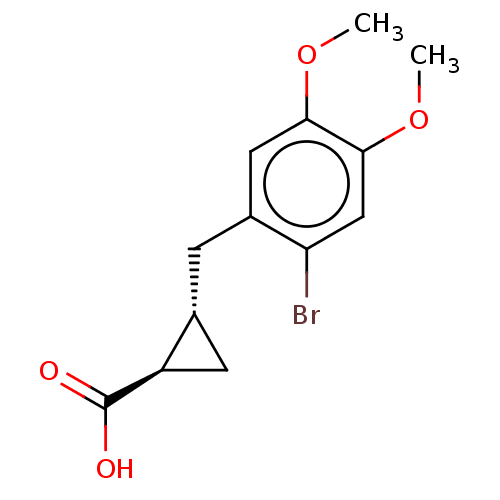
(CHEMBL4072131)Show SMILES COc1cc(Br)c(C[C@@H]2C[C@H]2C(O)=O)cc1OC |r| Show InChI InChI=1S/C13H15BrO4/c1-17-11-5-8(10(14)6-12(11)18-2)3-7-4-9(7)13(15)16/h5-7,9H,3-4H2,1-2H3,(H,15,16)/t7-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 by stopped-flow CO2 hydration method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233541

(CHEMBL4103210)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1cc(OC)c(OC)cc1Br |r| Show InChI InChI=1S/C15H19BrO4/c1-4-20-15(17)11-6-9(11)5-10-7-13(18-2)14(19-3)8-12(10)16/h7-9,11H,4-6H2,1-3H3/t9-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233536

(CHEMBL4090334)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C15H20O4/c1-4-19-15(16)12-9-11(12)7-10-5-6-13(17-2)14(8-10)18-3/h5-6,8,11-12H,4,7,9H2,1-3H3/t11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233550

(CHEMBL4063516)Show SMILES COc1cc(C[C@H]2C[C@H]2C(O)=O)c(Br)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-9-5-7(3-6-4-8(6)13(16)17)10(14)11(15)12(9)19-2/h5-6,8H,3-4H2,1-2H3,(H,16,17)/t6-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233545

(CHEMBL4105642)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1c(Br)c(Br)c(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H17Br3O4/c1-4-22-15(19)8-5-7(8)6-9-10(16)12(18)14(21-3)13(20-2)11(9)17/h7-8H,4-6H2,1-3H3/t7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233595

(CHEMBL4082617)Show SMILES COc1cc(C[C@@H]2C[C@H]2C(O)=O)c(Br)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-9-5-7(3-6-4-8(6)13(16)17)10(14)11(15)12(9)19-2/h5-6,8H,3-4H2,1-2H3,(H,16,17)/t6-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233548

(CHEMBL4064334)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1c(Br)c(Br)c(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H17Br3O4/c1-4-22-15(19)8-5-7(8)6-9-10(16)12(18)14(21-3)13(20-2)11(9)17/h7-8H,4-6H2,1-3H3/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233544

(CHEMBL4065358)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1c(Br)cc(OC)c(OC)c1Br |r| Show InChI InChI=1S/C15H18Br2O4/c1-4-21-15(18)9-5-8(9)6-10-11(16)7-12(19-2)14(20-3)13(10)17/h7-9H,4-6H2,1-3H3/t8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233549

(CHEMBL4092055)Show InChI InChI=1S/C13H15BrO4/c1-17-11-5-8(10(14)6-12(11)18-2)3-7-4-9(7)13(15)16/h5-7,9H,3-4H2,1-2H3,(H,15,16)/t7-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233546

(CHEMBL4087756)Show SMILES CCOC(=O)[C@@H]1C[C@H]1Cc1cc(OC)c(OC)cc1Br |r| Show InChI InChI=1S/C15H19BrO4/c1-4-20-15(17)11-6-9(11)5-10-7-13(18-2)14(19-3)8-12(10)16/h7-9,11H,4-6H2,1-3H3/t9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233540
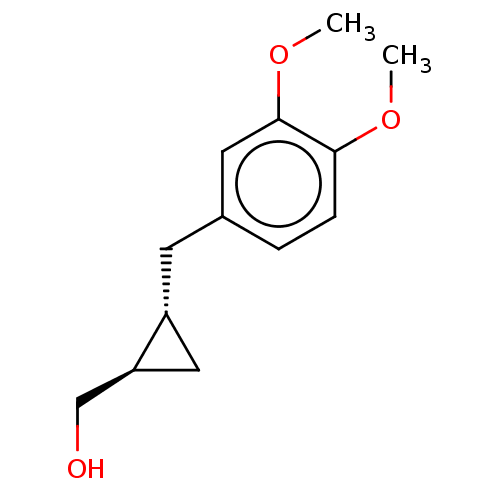
(CHEMBL4078936)Show InChI InChI=1S/C13H18O3/c1-15-12-4-3-9(6-13(12)16-2)5-10-7-11(10)8-14/h3-4,6,10-11,14H,5,7-8H2,1-2H3/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50233542

(CHEMBL4070958)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C15H20O4/c1-4-19-15(16)12-9-11(12)7-10-5-6-13(17-2)14(8-10)18-3/h5-6,8,11-12H,4,7,9H2,1-3H3/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233597

(CHEMBL4091363)Show SMILES COc1cc(Br)c(C[C@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H14Br2O4/c1-18-10-5-9(14)8(11(15)12(10)19-2)4-6-3-7(6)13(16)17/h5-7H,3-4H2,1-2H3,(H,16,17)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233598

(CHEMBL4072131)Show SMILES COc1cc(Br)c(C[C@@H]2C[C@H]2C(O)=O)cc1OC |r| Show InChI InChI=1S/C13H15BrO4/c1-17-11-5-8(10(14)6-12(11)18-2)3-7-4-9(7)13(15)16/h5-7,9H,3-4H2,1-2H3,(H,15,16)/t7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233593

(CHEMBL4069416)Show SMILES COc1c(Br)c(Br)c(C[C@@H]2C[C@H]2C(O)=O)c(Br)c1OC |r| Show InChI InChI=1S/C13H13Br3O4/c1-19-11-9(15)7(4-5-3-6(5)13(17)18)8(14)10(16)12(11)20-2/h5-6H,3-4H2,1-2H3,(H,17,18)/t5-,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233543

(CHEMBL4089410)Show InChI InChI=1S/C13H18O3/c1-15-12-4-3-9(6-13(12)16-2)5-10-7-11(10)8-14/h3-4,6,10-11,14H,5,7-8H2,1-2H3/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233540

(CHEMBL4078936)Show InChI InChI=1S/C13H18O3/c1-15-12-4-3-9(6-13(12)16-2)5-10-7-11(10)8-14/h3-4,6,10-11,14H,5,7-8H2,1-2H3/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50233539

(CHEMBL4068228)Show InChI InChI=1S/C13H16O4/c1-16-11-4-3-8(6-12(11)17-2)5-9-7-10(9)13(14)15/h3-4,6,9-10H,5,7H2,1-2H3,(H,14,15)/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 by stopped-flow CO2 hydration method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using para-nitrophenylacetate as substrate measured over 3 mins by UV-vis spectrophotometric method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50233541

(CHEMBL4103210)Show SMILES CCOC(=O)[C@@H]1C[C@@H]1Cc1cc(OC)c(OC)cc1Br |r| Show InChI InChI=1S/C15H19BrO4/c1-4-20-15(17)11-6-9(11)5-10-7-13(18-2)14(19-3)8-12(10)16/h7-9,11H,4-6H2,1-3H3/t9-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 by stopped-flow CO2 hydration method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50233549

(CHEMBL4092055)Show InChI InChI=1S/C13H15BrO4/c1-17-11-5-8(10(14)6-12(11)18-2)3-7-4-9(7)13(15)16/h5-7,9H,3-4H2,1-2H3,(H,15,16)/t7-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 by stopped-flow CO2 hydration method |
J Med Chem 58: 640-50 (2015)
Article DOI: 10.1021/jm501573b
BindingDB Entry DOI: 10.7270/Q2PR7Z6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data