

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355862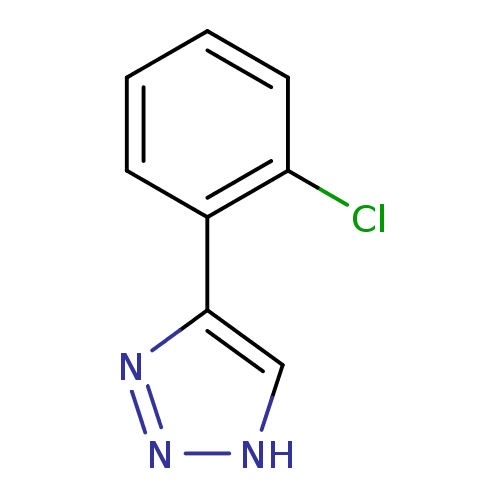 (CHEMBL1909734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate by Dixon... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355861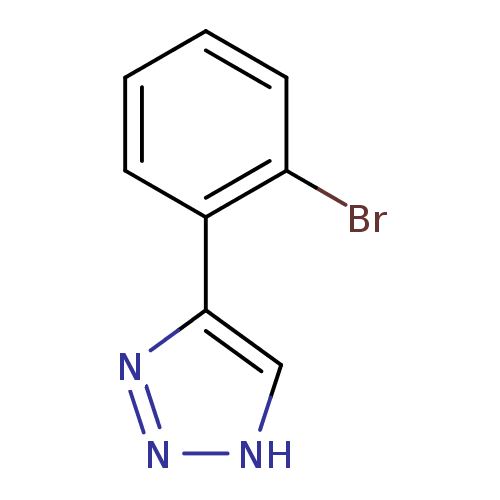 (CHEMBL1909733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate by Dixon... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17448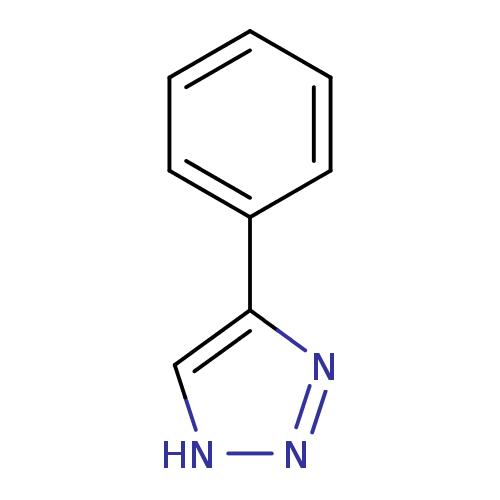 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate by Dixon... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973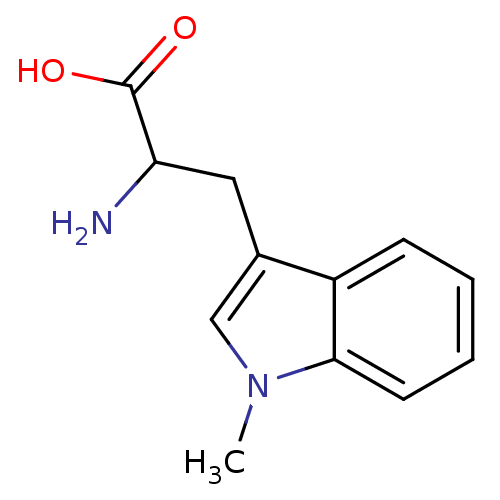 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Competitive inhibition of indoleamine-2,3-dioxygenase | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355862 (CHEMBL1909734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of indoleamine-2,3-dioxygenase in human HEK293 cells assessed as N-formylkynurenine level after 5 hrs by spectrophotometric analysis | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355861 (CHEMBL1909733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of indoleamine-2,3-dioxygenase in human HEK293 cells assessed as N-formylkynurenine level after 5 hrs by spectrophotometric analysis | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355862 (CHEMBL1909734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17448 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355861 (CHEMBL1909733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17448 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of indoleamine-2,3-dioxygenase in human HEK293 cells assessed as N-formylkynurenine level after 5 hrs by spectrophotometric analysis | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355865 (CHEMBL1909741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355867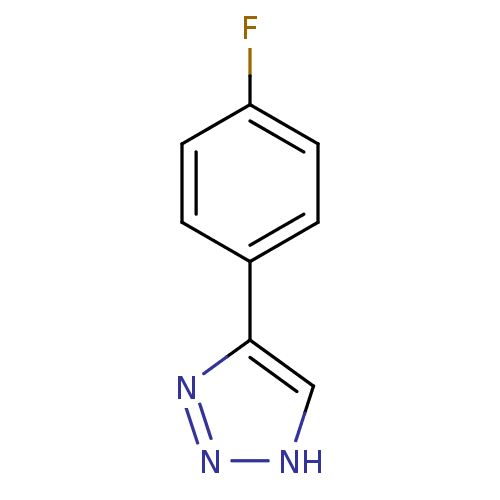 (CHEMBL1909735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17471 (1,2,3-triazole analogue, 27 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17459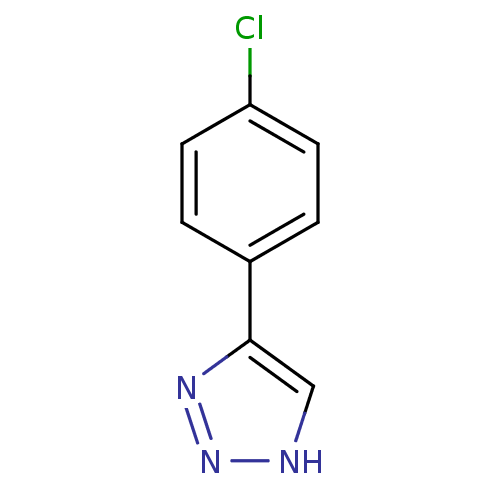 (1,2,3-triazole analogue, 15 | 5-(4-chlorophenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17453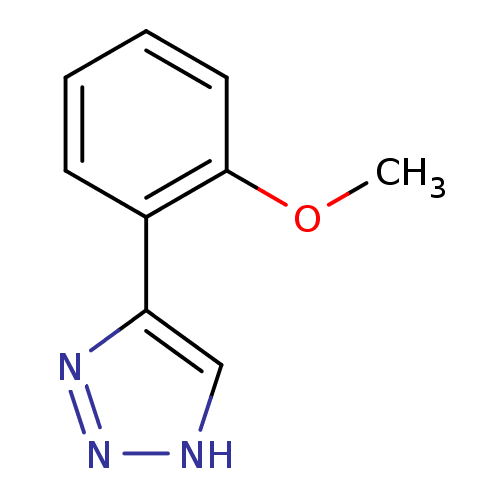 (1,2,3-triazole analogue, 9 | 5-(2-methoxyphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355864 (CHEMBL1909740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.03E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17460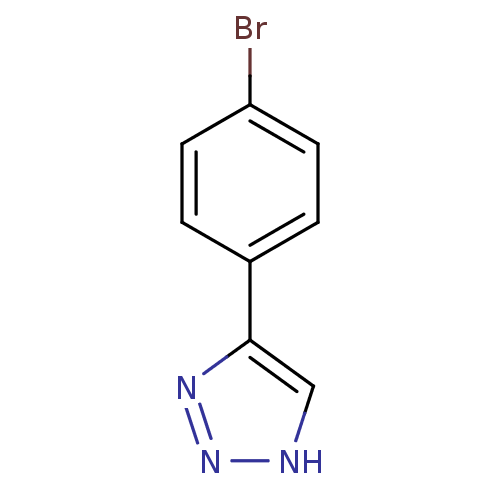 (1,2,3-triazole analogue, 16 | 5-(4-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355863 (CHEMBL1909739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.37E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355866 (CHEMBL1909742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM36293 (1,2,3-benzotriazole | Benzotriazole (Bta)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.57E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355860 (CHEMBL1909732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro... | Eur J Med Chem 46: 5680-7 (2011) Article DOI: 10.1016/j.ejmech.2011.08.044 BindingDB Entry DOI: 10.7270/Q2KH0NRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||