 Found 111 hits Enz. Inhib. hit(s) with all data for entry = 50034088
Found 111 hits Enz. Inhib. hit(s) with all data for entry = 50034088 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356873
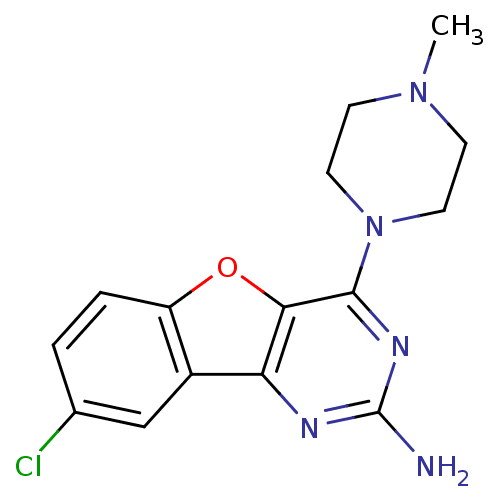
(CHEMBL1914541)Show InChI InChI=1S/C15H16ClN5O/c1-20-4-6-21(7-5-20)14-13-12(18-15(17)19-14)10-8-9(16)2-3-11(10)22-13/h2-3,8H,4-7H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356794
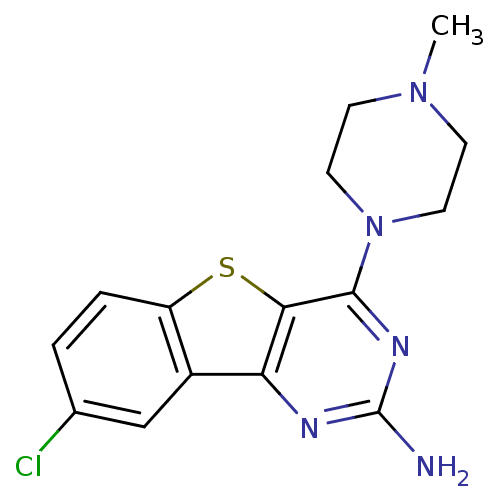
(CHEMBL1914462)Show InChI InChI=1S/C15H16ClN5S/c1-20-4-6-21(7-5-20)14-13-12(18-15(17)19-14)10-8-9(16)2-3-11(10)22-13/h2-3,8H,4-7H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315348
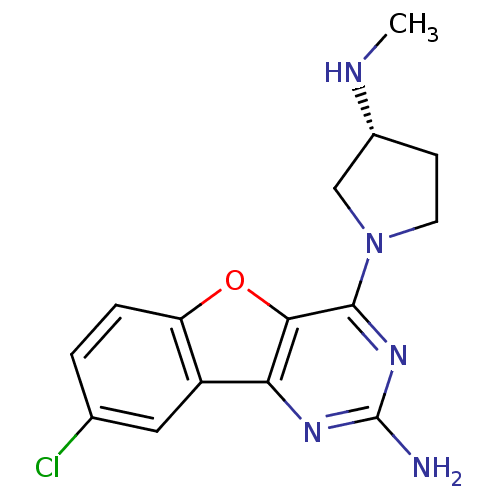
((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3oc12 |r| Show InChI InChI=1S/C15H16ClN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356815

(CHEMBL1914781)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Br)ccc3oc12 |r| Show InChI InChI=1S/C15H16BrN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356788
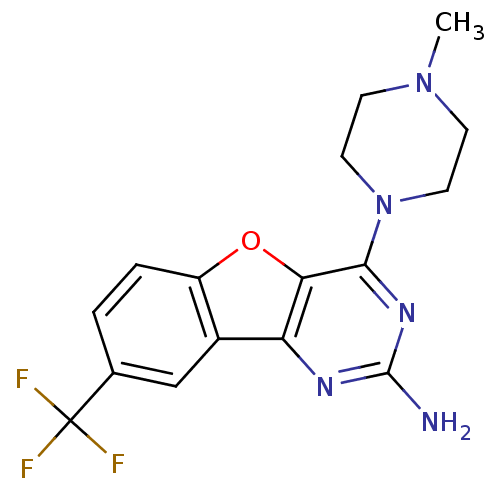
(CHEMBL1914755)Show SMILES CN1CCN(CC1)c1nc(N)nc2c3cc(ccc3oc12)C(F)(F)F Show InChI InChI=1S/C16H16F3N5O/c1-23-4-6-24(7-5-23)14-13-12(21-15(20)22-14)10-8-9(16(17,18)19)2-3-11(10)25-13/h2-3,8H,4-7H2,1H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315348

((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3oc12 |r| Show InChI InChI=1S/C15H16ClN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356793
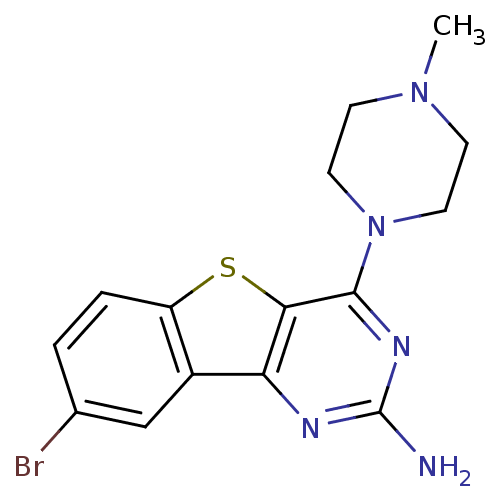
(CHEMBL1914760)Show InChI InChI=1S/C15H16BrN5S/c1-20-4-6-21(7-5-20)14-13-12(18-15(17)19-14)10-8-9(16)2-3-11(10)22-13/h2-3,8H,4-7H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356817

(CHEMBL1914783)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(ccc3oc12)C(F)(F)F |r| Show InChI InChI=1S/C16H16F3N5O/c1-21-9-4-5-24(7-9)14-13-12(22-15(20)23-14)10-6-8(16(17,18)19)2-3-11(10)25-13/h2-3,6,9,21H,4-5,7H2,1H3,(H2,20,22,23)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356872
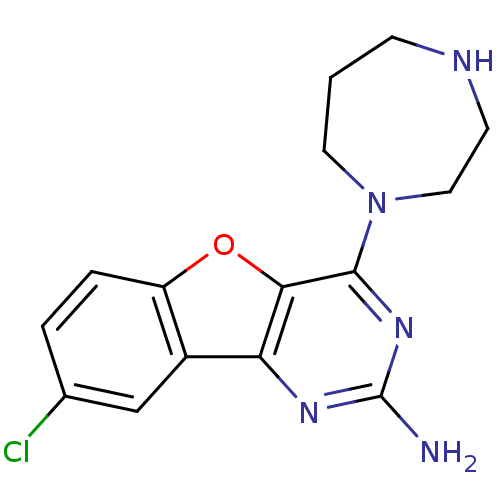
(CHEMBL1914540)Show InChI InChI=1S/C15H16ClN5O/c16-9-2-3-11-10(8-9)12-13(22-11)14(20-15(17)19-12)21-6-1-4-18-5-7-21/h2-3,8,18H,1,4-7H2,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356874
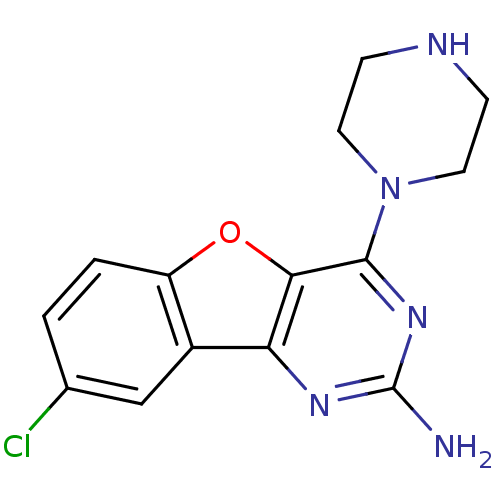
(CHEMBL1914542)Show InChI InChI=1S/C14H14ClN5O/c15-8-1-2-10-9(7-8)11-12(21-10)13(19-14(16)18-11)20-5-3-17-4-6-20/h1-2,7,17H,3-6H2,(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356816

(CHEMBL1914782)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(F)ccc3oc12 |r| Show InChI InChI=1S/C15H16FN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315349
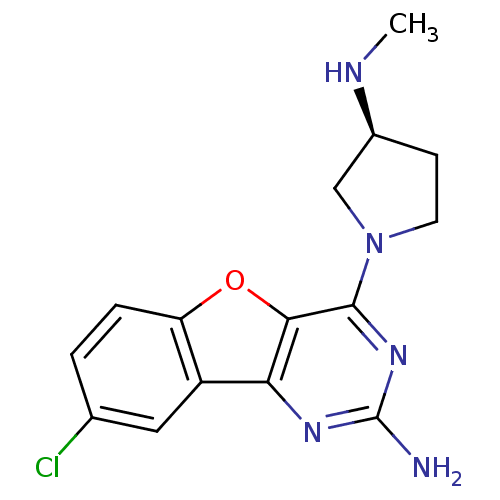
((S)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...)Show SMILES CN[C@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3oc12 |r| Show InChI InChI=1S/C15H16ClN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356822

(CHEMBL1914788)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Br)ccc3sc12 |r| Show InChI InChI=1S/C15H16BrN5S/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356771
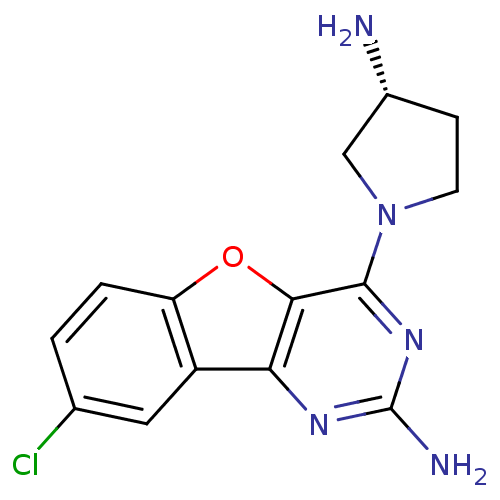
(CHEMBL1914543)Show SMILES N[C@@H]1CCN(C1)c1nc(N)nc2c1oc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C14H14ClN5O/c15-7-1-2-10-9(5-7)11-12(21-10)13(19-14(17)18-11)20-4-3-8(16)6-20/h1-2,5,8H,3-4,6,16H2,(H2,17,18,19)/t8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356810
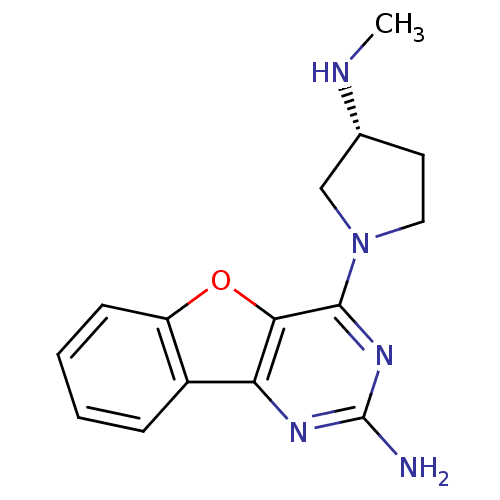
(CHEMBL1914776)Show InChI InChI=1S/C15H17N5O/c1-17-9-6-7-20(8-9)14-13-12(18-15(16)19-14)10-4-2-3-5-11(10)21-13/h2-5,9,17H,6-8H2,1H3,(H2,16,18,19)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356775

(CHEMBL1914547)Show InChI InChI=1S/C14H14ClN5O/c15-8-1-2-10-9(3-8)11-12(21-10)13(19-14(17)18-11)20-5-7(4-16)6-20/h1-3,7H,4-6,16H2,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
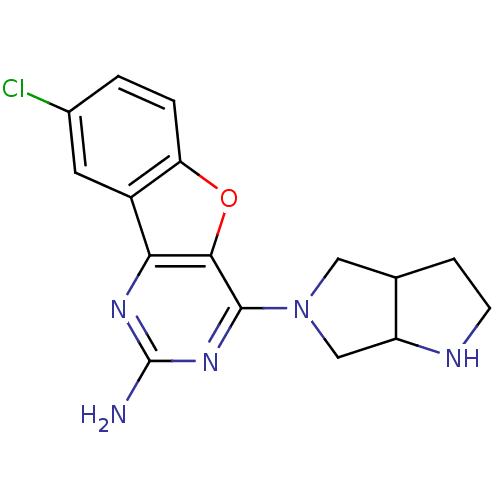
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356779
(CHEMBL1914745)Show InChI InChI=1S/C16H16ClN5O/c17-9-1-2-12-10(5-9)13-14(23-12)15(21-16(18)20-13)22-6-8-3-4-19-11(8)7-22/h1-2,5,8,11,19H,3-4,6-7H2,(H2,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356780

(CHEMBL1914746)Show SMILES Nc1nc(N2C[C@H]3CCCN[C@H]3C2)c2oc3ccc(Cl)cc3c2n1 |r| Show InChI InChI=1S/C17H18ClN5O/c18-10-3-4-13-11(6-10)14-15(24-13)16(22-17(19)21-14)23-7-9-2-1-5-20-12(9)8-23/h3-4,6,9,12,20H,1-2,5,7-8H2,(H2,19,21,22)/t9-,12+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315314
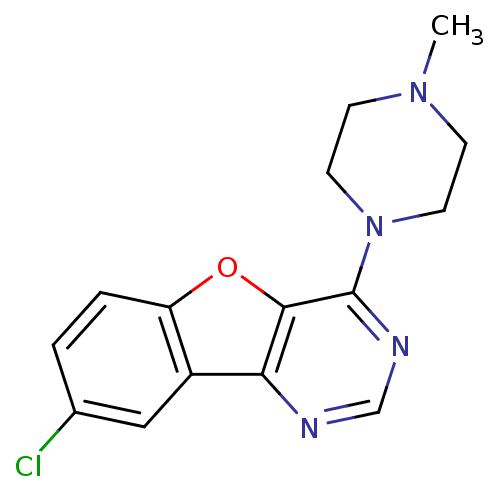
(8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...)Show InChI InChI=1S/C15H15ClN4O/c1-19-4-6-20(7-5-19)15-14-13(17-9-18-15)11-8-10(16)2-3-12(11)21-14/h2-3,8-9H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356875
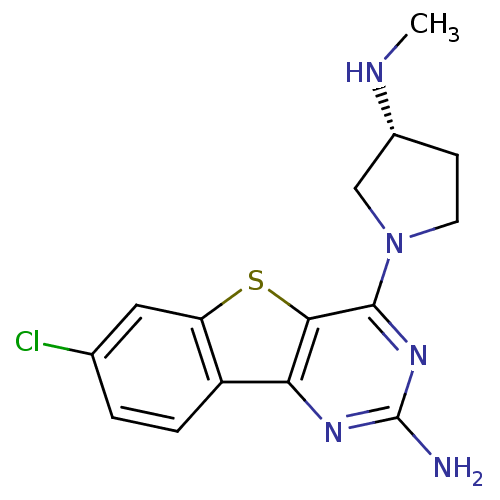
(CHEMBL1914792)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3ccc(Cl)cc3sc12 |r| Show InChI InChI=1S/C15H16ClN5S/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-3-2-8(16)6-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356776

(CHEMBL1914548)Show SMILES N[C@H]1CCCN(C1)c1nc(N)nc2c1oc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C15H16ClN5O/c16-8-3-4-11-10(6-8)12-13(22-11)14(20-15(18)19-12)21-5-1-2-9(17)7-21/h3-4,6,9H,1-2,5,7,17H2,(H2,18,19,20)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
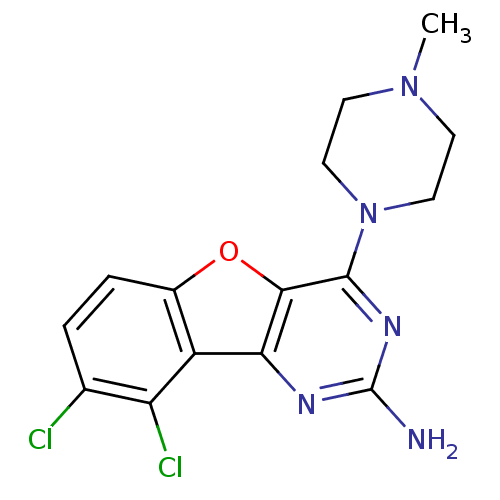
(Homo sapiens (Human)) | BDBM50356790
(CHEMBL1914757)Show InChI InChI=1S/C15H15Cl2N5O/c1-21-4-6-22(7-5-21)14-13-12(19-15(18)20-14)10-9(23-13)3-2-8(16)11(10)17/h2-3H,4-7H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356808

(CHEMBL1914774)Show InChI InChI=1S/C14H13Cl2N5O/c15-7-1-2-8-9(10(7)16)11-12(22-8)13(20-14(17)19-11)21-5-3-18-4-6-21/h1-2,18H,3-6H2,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356819

(CHEMBL1914785)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)cc(Cl)c3oc12 |r| Show InChI InChI=1S/C15H15Cl2N5O/c1-19-8-2-3-22(6-8)14-13-11(20-15(18)21-14)9-4-7(16)5-10(17)12(9)23-13/h4-5,8,19H,2-3,6H2,1H3,(H2,18,20,21)/t8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356777
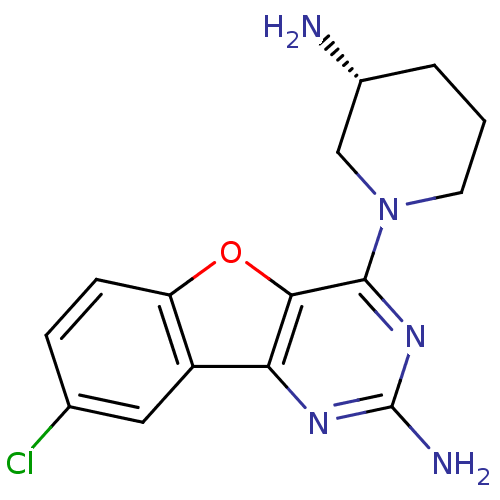
(CHEMBL1914549)Show SMILES N[C@@H]1CCCN(C1)c1nc(N)nc2c1oc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C15H16ClN5O/c16-8-3-4-11-10(6-8)12-13(22-11)14(20-15(18)19-12)21-5-1-2-9(17)7-21/h3-4,6,9H,1-2,5,7,17H2,(H2,18,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356859
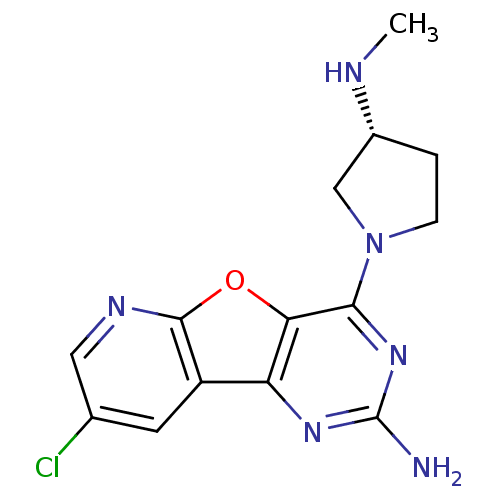
(CHEMBL1915048)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)cnc3oc12 |r| Show InChI InChI=1S/C14H15ClN6O/c1-17-8-2-3-21(6-8)12-11-10(19-14(16)20-12)9-4-7(15)5-18-13(9)22-11/h4-5,8,17H,2-3,6H2,1H3,(H2,16,19,20)/t8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356812
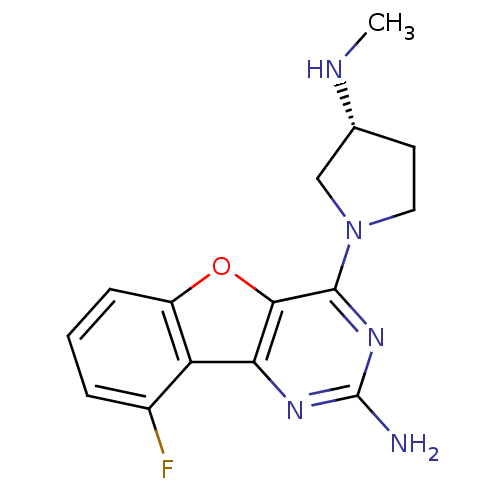
(CHEMBL1914778)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c1oc1cccc(F)c21 |r| Show InChI InChI=1S/C15H16FN5O/c1-18-8-5-6-21(7-8)14-13-12(19-15(17)20-14)11-9(16)3-2-4-10(11)22-13/h2-4,8,18H,5-7H2,1H3,(H2,17,19,20)/t8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356820

(CHEMBL1914786)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c1oc1ccc(Cl)c(Cl)c21 |r| Show InChI InChI=1S/C15H15Cl2N5O/c1-19-7-4-5-22(6-7)14-13-12(20-15(18)21-14)10-9(23-13)3-2-8(16)11(10)17/h2-3,7,19H,4-6H2,1H3,(H2,18,20,21)/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356823

(CHEMBL1914789)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3sc12 |r| Show InChI InChI=1S/C15H16ClN5S/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356818

(CHEMBL1914784)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(OC)ccc3oc12 |r| Show InChI InChI=1S/C16H19N5O2/c1-18-9-5-6-21(8-9)15-14-13(19-16(17)20-15)11-7-10(22-2)3-4-12(11)23-14/h3-4,7,9,18H,5-6,8H2,1-2H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356789

(CHEMBL1914756)Show InChI InChI=1S/C16H19N5O2/c1-20-5-7-21(8-6-20)15-14-13(18-16(17)19-15)11-9-10(22-2)3-4-12(11)23-14/h3-4,9H,5-8H2,1-2H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356806

(CHEMBL1914772)Show InChI InChI=1S/C15H14F3N5O/c16-15(17,18)8-1-2-10-9(7-8)11-12(24-10)13(22-14(19)21-11)23-5-3-20-4-6-23/h1-2,7,20H,3-6H2,(H2,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356860

(CHEMBL1915049)Show SMILES N[C@@H]1CCN(C1)c1nc(N)nc2c1oc1ncc(Cl)cc21 |r| Show InChI InChI=1S/C13H13ClN6O/c14-6-3-8-9-10(21-12(8)17-4-6)11(19-13(16)18-9)20-2-1-7(15)5-20/h3-4,7H,1-2,5,15H2,(H2,16,18,19)/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356797

(CHEMBL1914763)Show InChI InChI=1S/C15H16ClN5S/c1-20-4-6-21(7-5-20)14-13-12(18-15(17)19-14)10-3-2-9(16)8-11(10)22-13/h2-3,8H,4-7H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356774
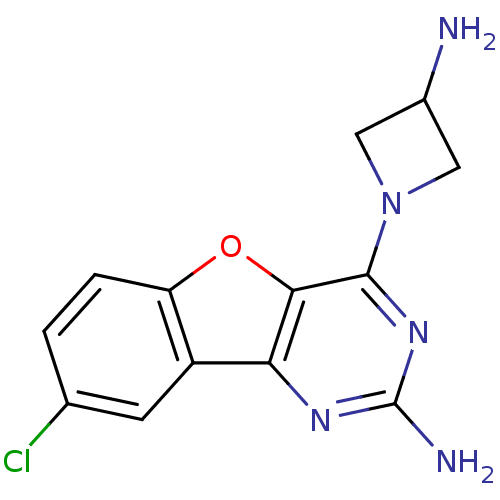
(CHEMBL1914546)Show InChI InChI=1S/C13H12ClN5O/c14-6-1-2-9-8(3-6)10-11(20-9)12(18-13(16)17-10)19-4-7(15)5-19/h1-3,7H,4-5,15H2,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356795

(CHEMBL1914761)Show InChI InChI=1S/C15H16FN5S/c1-20-4-6-21(7-5-20)14-13-12(18-15(17)19-14)10-8-9(16)2-3-11(10)22-13/h2-3,8H,4-7H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356814
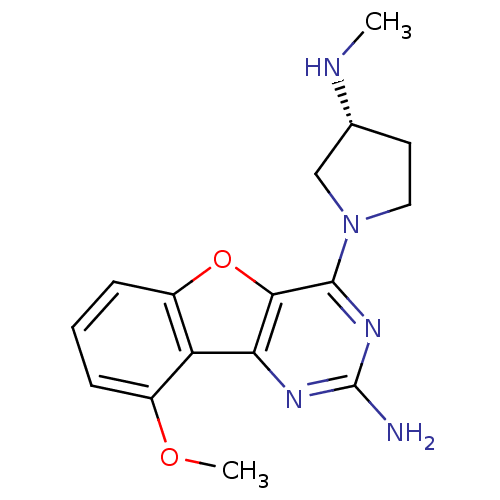
(CHEMBL1914780)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c1oc1cccc(OC)c21 |r| Show InChI InChI=1S/C16H19N5O2/c1-18-9-6-7-21(8-9)15-14-13(19-16(17)20-15)12-10(22-2)4-3-5-11(12)23-14/h3-5,9,18H,6-8H2,1-2H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356801

(CHEMBL1914767)Show InChI InChI=1S/C14H15N5O/c15-14-17-11-9-3-1-2-4-10(9)20-12(11)13(18-14)19-7-5-16-6-8-19/h1-4,16H,5-8H2,(H2,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356785
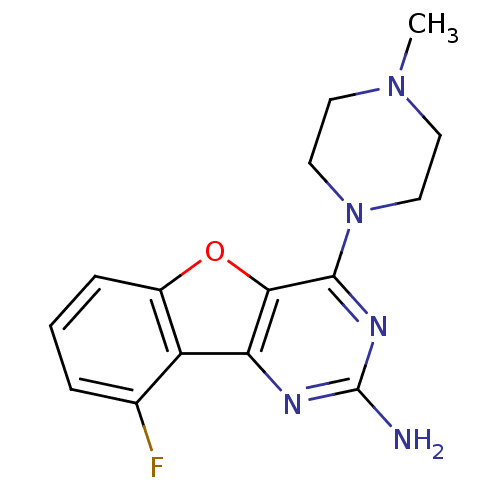
(CHEMBL1914752)Show InChI InChI=1S/C15H16FN5O/c1-20-5-7-21(8-6-20)14-13-12(18-15(17)19-14)11-9(16)3-2-4-10(11)22-13/h2-4H,5-8H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356781

(CHEMBL1914747)Show SMILES Nc1nc(N2C[C@@H]3CCCN[C@@H]3C2)c2oc3ccc(Cl)cc3c2n1 |r| Show InChI InChI=1S/C17H18ClN5O/c18-10-3-4-13-11(6-10)14-15(24-13)16(22-17(19)21-14)23-7-9-2-1-5-20-12(9)8-23/h3-4,6,9,12,20H,1-2,5,7-8H2,(H2,19,21,22)/t9-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356825
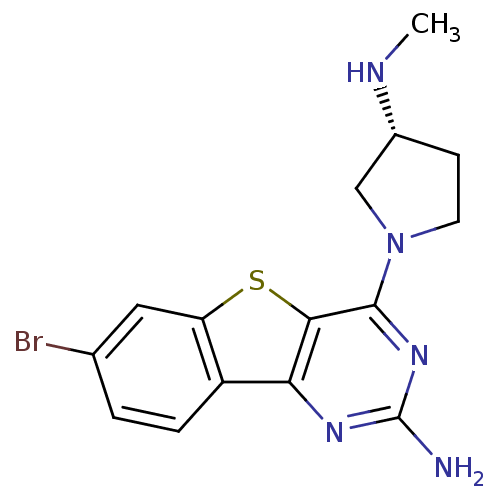
(CHEMBL1914791)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3ccc(Br)cc3sc12 |r| Show InChI InChI=1S/C15H16BrN5S/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-3-2-8(16)6-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356782
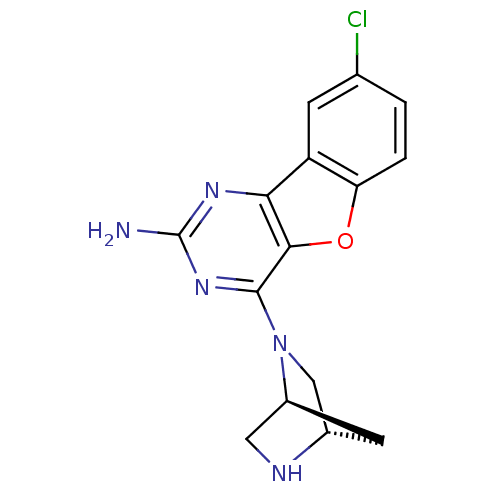
(CHEMBL1914748)Show SMILES Nc1nc(N2C[C@@H]3C[C@H]2CN3)c2oc3ccc(Cl)cc3c2n1 |r| Show InChI InChI=1S/C15H14ClN5O/c16-7-1-2-11-10(3-7)12-13(22-11)14(20-15(17)19-12)21-6-8-4-9(21)5-18-8/h1-3,8-9,18H,4-6H2,(H2,17,19,20)/t8-,9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
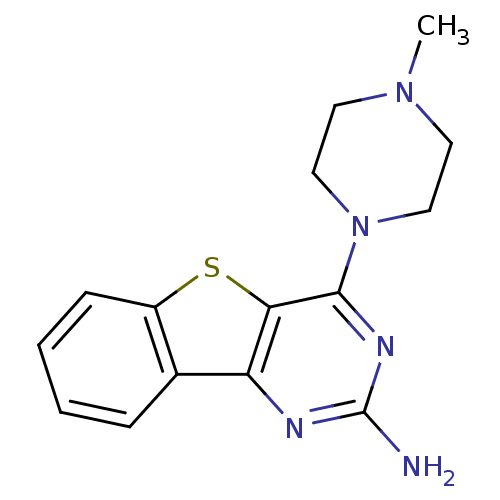
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356792
(CHEMBL1914759)Show InChI InChI=1S/C15H17N5S/c1-19-6-8-20(9-7-19)14-13-12(17-15(16)18-14)10-4-2-3-5-11(10)21-13/h2-5H,6-9H2,1H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
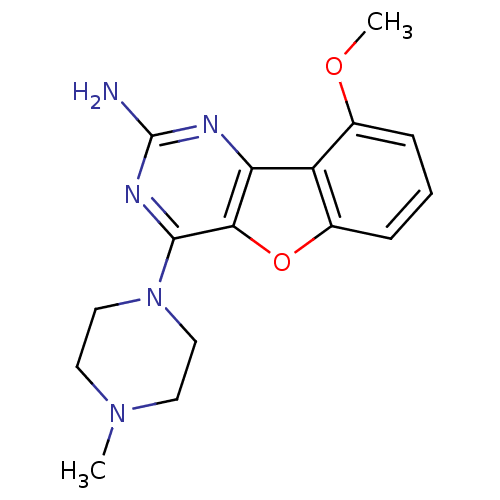
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356787
(CHEMBL1914754)Show InChI InChI=1S/C16H19N5O2/c1-20-6-8-21(9-7-20)15-14-13(18-16(17)19-15)12-10(22-2)4-3-5-11(12)23-14/h3-5H,6-9H2,1-2H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356824
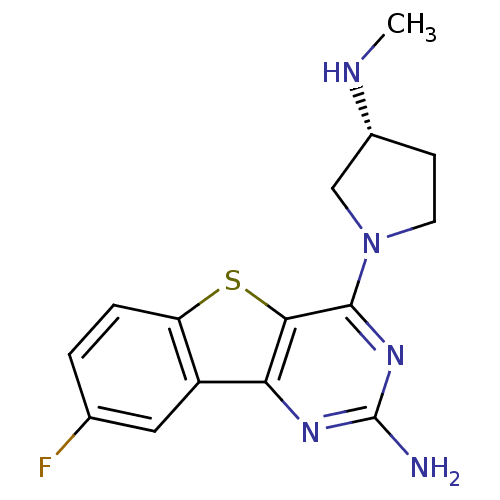
(CHEMBL1914790)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(F)ccc3sc12 |r| Show InChI InChI=1S/C15H16FN5S/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356831
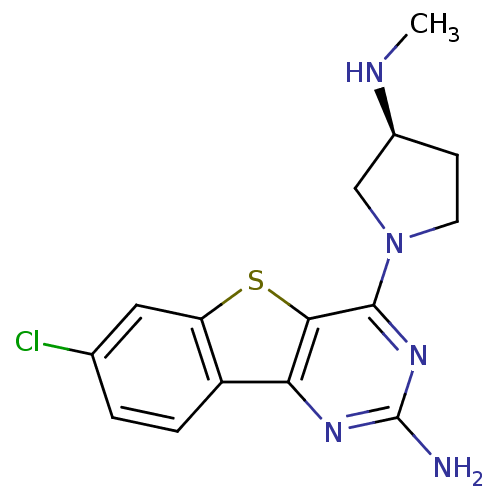
(CHEMBL1915020)Show SMILES CN[C@H]1CCN(C1)c1nc(N)nc2c3ccc(Cl)cc3sc12 |r| Show InChI InChI=1S/C15H16ClN5S/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-3-2-8(16)6-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356855

(CHEMBL1915044)Show InChI InChI=1S/C14H15ClN6O/c1-20-2-4-21(5-3-20)12-11-10(18-14(16)19-12)9-6-8(15)7-17-13(9)22-11/h6-7H,2-5H2,1H3,(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356856
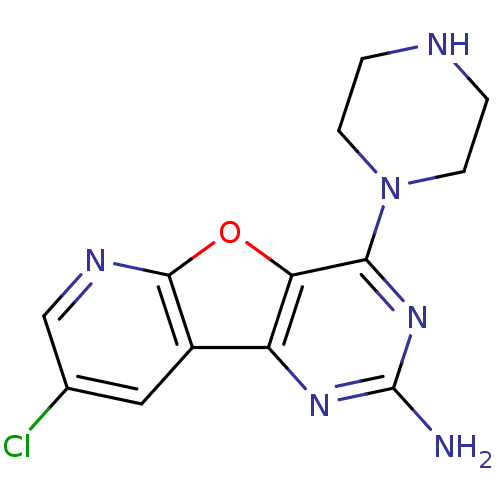
(CHEMBL1915045)Show InChI InChI=1S/C13H13ClN6O/c14-7-5-8-9-10(21-12(8)17-6-7)11(19-13(15)18-9)20-3-1-16-2-4-20/h5-6,16H,1-4H2,(H2,15,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
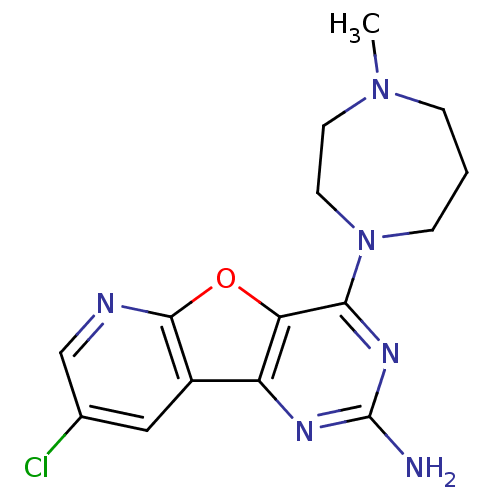
(Homo sapiens (Human)) | BDBM50356858
(CHEMBL1915047)Show InChI InChI=1S/C15H17ClN6O/c1-21-3-2-4-22(6-5-21)13-12-11(19-15(17)20-13)10-7-9(16)8-18-14(10)23-12/h7-8H,2-6H2,1H3,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356821

(CHEMBL1914787)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c1oc1ccc(F)c(F)c21 |r| Show InChI InChI=1S/C15H15F2N5O/c1-19-7-4-5-22(6-7)14-13-12(20-15(18)21-14)10-9(23-13)3-2-8(16)11(10)17/h2-3,7,19H,4-6H2,1H3,(H2,18,20,21)/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data