 Found 60 hits Enz. Inhib. hit(s) with all data for entry = 50034383
Found 60 hits Enz. Inhib. hit(s) with all data for entry = 50034383 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346873
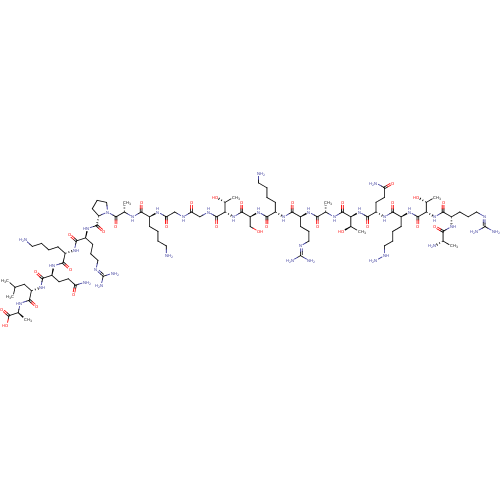
(CHEMBL1797652)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C94H173N37O28/c1-46(2)42-63(84(151)116-50(6)91(158)159)126-81(148)61(30-32-66(99)136)122-76(143)55(23-11-15-35-96)120-79(146)59(27-19-38-109-93(103)104)124-86(153)65-29-21-41-131(65)90(157)49(5)115-75(142)54(22-10-14-34-95)117-69(139)44-111-68(138)43-112-87(154)70(51(7)133)128-85(152)64(45-132)127-80(147)56(24-12-16-36-97)121-78(145)58(26-18-37-108-92(101)102)119-74(141)48(4)114-88(155)71(52(8)134)129-83(150)62(31-33-67(100)137)123-77(144)57(25-13-17-40-113-107)125-89(156)72(53(9)135)130-82(149)60(118-73(140)47(3)98)28-20-39-110-94(105)106/h46-65,70-72,113,132-135H,10-45,95-98,107H2,1-9H3,(H2,99,136)(H2,100,137)(H,111,138)(H,112,154)(H,114,155)(H,115,142)(H,116,151)(H,117,139)(H,118,140)(H,119,141)(H,120,146)(H,121,145)(H,122,143)(H,123,144)(H,124,153)(H,125,156)(H,126,148)(H,127,147)(H,128,152)(H,129,150)(H,130,149)(H,158,159)(H4,101,102,108)(H4,103,104,109)(H4,105,106,110)/t47-,48-,49-,50-,51+,52+,53+,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,70-,71-,72-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361478
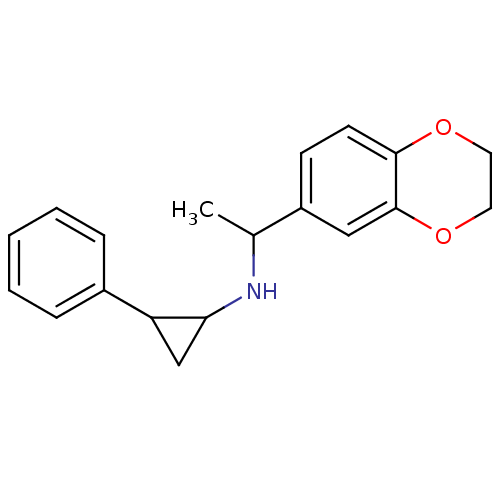
(CHEMBL1938897)Show InChI InChI=1S/C19H21NO2/c1-13(15-7-8-18-19(11-15)22-10-9-21-18)20-17-12-16(17)14-5-3-2-4-6-14/h2-8,11,13,16-17,20H,9-10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361481
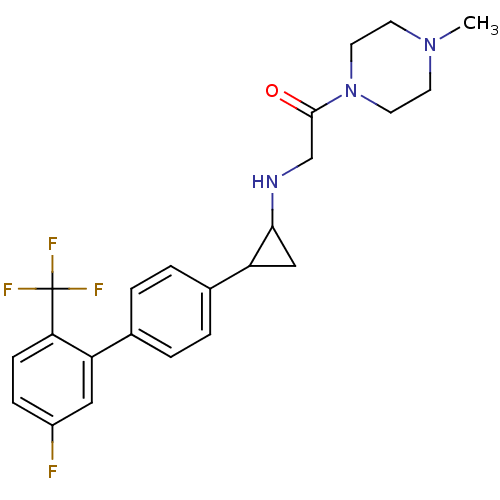
(CHEMBL1938898)Show SMILES CN1CCN(CC1)C(=O)CNC1CC1c1ccc(cc1)-c1cc(F)ccc1C(F)(F)F Show InChI InChI=1S/C23H25F4N3O/c1-29-8-10-30(11-9-29)22(31)14-28-21-13-19(21)16-4-2-15(3-5-16)18-12-17(24)6-7-20(18)23(25,26)27/h2-7,12,19,21,28H,8-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346875
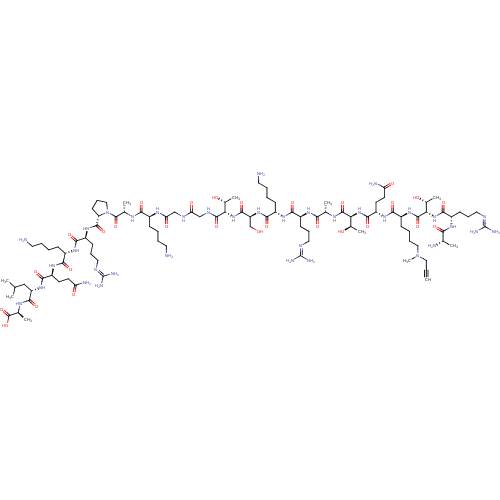
(CHEMBL1797648)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7](-[#6])-[#6]C#C)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C98H176N36O28/c1-12-43-133(11)44-20-16-28-61(127-93(159)76(57(10)138)132-86(152)64(120-77(143)51(4)102)31-23-42-113-98(109)110)81(147)125-66(34-36-71(104)140)87(153)131-75(56(9)137)92(158)116-52(5)78(144)121-62(29-21-40-111-96(105)106)82(148)123-60(27-15-19-39-101)84(150)129-68(49-135)89(155)130-74(55(8)136)91(157)115-47-72(141)114-48-73(142)119-58(25-13-17-37-99)79(145)117-53(6)94(160)134-45-24-32-69(134)90(156)126-63(30-22-41-112-97(107)108)83(149)122-59(26-14-18-38-100)80(146)124-65(33-35-70(103)139)85(151)128-67(46-50(2)3)88(154)118-54(7)95(161)162/h1,50-69,74-76,135-138H,13-49,99-102H2,2-11H3,(H2,103,139)(H2,104,140)(H,114,141)(H,115,157)(H,116,158)(H,117,145)(H,118,154)(H,119,142)(H,120,143)(H,121,144)(H,122,149)(H,123,148)(H,124,146)(H,125,147)(H,126,156)(H,127,159)(H,128,151)(H,129,150)(H,130,155)(H,131,153)(H,132,152)(H,161,162)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,54-,55+,56+,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,74-,75-,76-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585
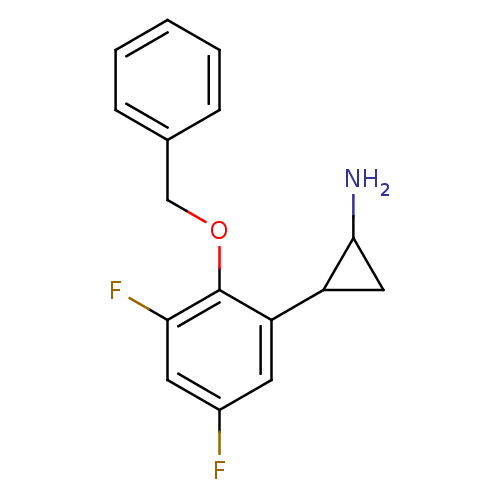
(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346864
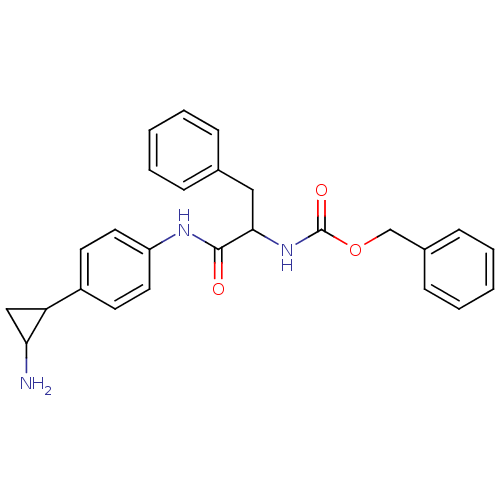
(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346864

(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346862
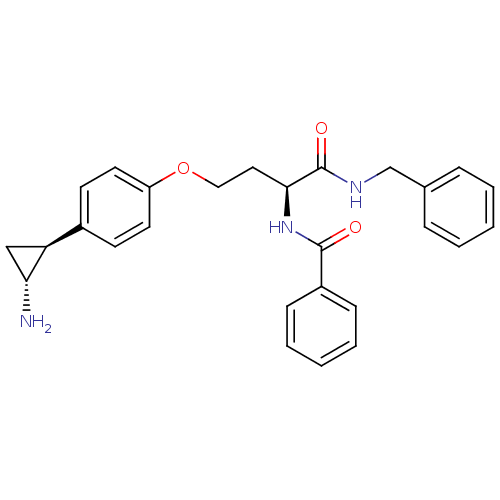
(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346589
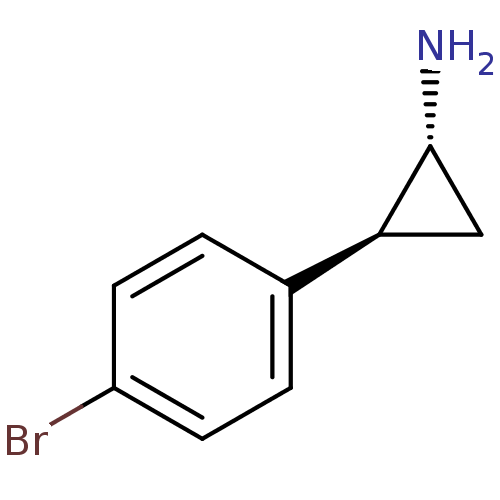
(CHEMBL1795974 | CHEMBL255520)Show InChI InChI=1S/C9H10BrN/c10-7-3-1-6(2-4-7)8-5-9(8)11/h1-4,8-9H,5,11H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346534
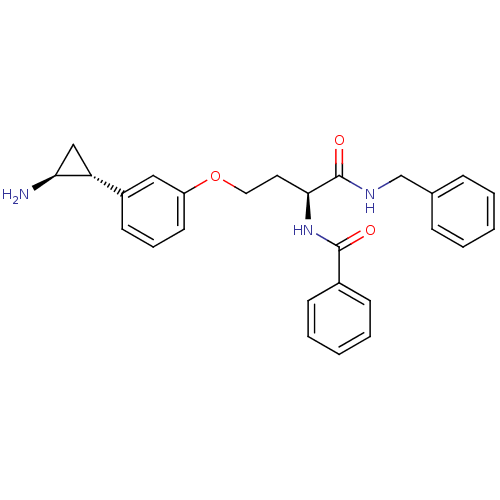
(CHEMBL1797705)Show SMILES N[C@H]1C[C@@H]1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50361478

(CHEMBL1938897)Show InChI InChI=1S/C19H21NO2/c1-13(15-7-8-18-19(11-15)22-10-9-21-18)20-17-12-16(17)14-5-3-2-4-6-14/h2-8,11,13,16-17,20H,9-10,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50361481

(CHEMBL1938898)Show SMILES CN1CCN(CC1)C(=O)CNC1CC1c1ccc(cc1)-c1cc(F)ccc1C(F)(F)F Show InChI InChI=1S/C23H25F4N3O/c1-29-8-10-30(11-9-29)22(31)14-28-21-13-19(21)16-4-2-15(3-5-16)18-12-17(24)6-7-20(18)23(25,26)27/h2-7,12,19,21,28H,8-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50361478

(CHEMBL1938897)Show InChI InChI=1S/C19H21NO2/c1-13(15-7-8-18-19(11-15)22-10-9-21-18)20-17-12-16(17)14-5-3-2-4-6-14/h2-8,11,13,16-17,20H,9-10,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50236898

((1S,2R)-(+)-2-phenylcyclopropylamine | CHEMBL25799...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50361479
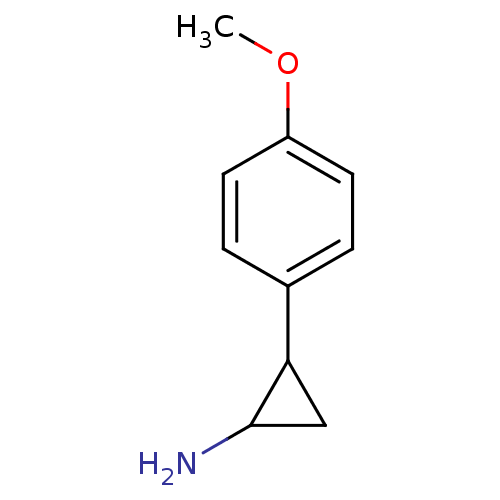
(CHEMBL1938899)Show InChI InChI=1S/C10H13NO/c1-12-8-4-2-7(3-5-8)9-6-10(9)11/h2-5,9-10H,6,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 2
(Homo sapiens (Human)) | BDBM50346864

(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD2 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50361481

(CHEMBL1938898)Show SMILES CN1CCN(CC1)C(=O)CNC1CC1c1ccc(cc1)-c1cc(F)ccc1C(F)(F)F Show InChI InChI=1S/C23H25F4N3O/c1-29-8-10-30(11-9-29)22(31)14-28-21-13-19(21)16-4-2-15(3-5-16)18-12-17(24)6-7-20(18)23(25,26)27/h2-7,12,19,21,28H,8-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50361479

(CHEMBL1938899)Show InChI InChI=1S/C10H13NO/c1-12-8-4-2-7(3-5-8)9-6-10(9)11/h2-5,9-10H,6,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50236898

((1S,2R)-(+)-2-phenylcyclopropylamine | CHEMBL25799...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50346864

(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361479

(CHEMBL1938899)Show InChI InChI=1S/C10H13NO/c1-12-8-4-2-7(3-5-8)9-6-10(9)11/h2-5,9-10H,6,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346534

(CHEMBL1797705)Show SMILES N[C@H]1C[C@@H]1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50236898

((1S,2R)-(+)-2-phenylcyclopropylamine | CHEMBL25799...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346862

(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50346534

(CHEMBL1797705)Show SMILES N[C@H]1C[C@@H]1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50346862

(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4C
(Homo sapiens (Human)) | BDBM50324535
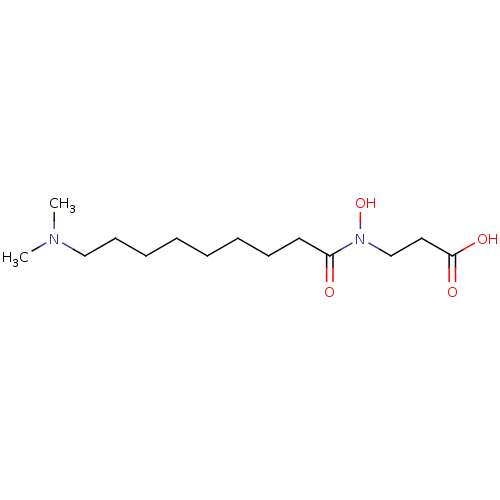
(3-{[9-(Dimethylamino)nonanoyl](hydroxy)amino}propa...)Show InChI InChI=1S/C14H28N2O4/c1-15(2)11-8-6-4-3-5-7-9-13(17)16(20)12-10-14(18)19/h20H,3-12H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4C |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50185388
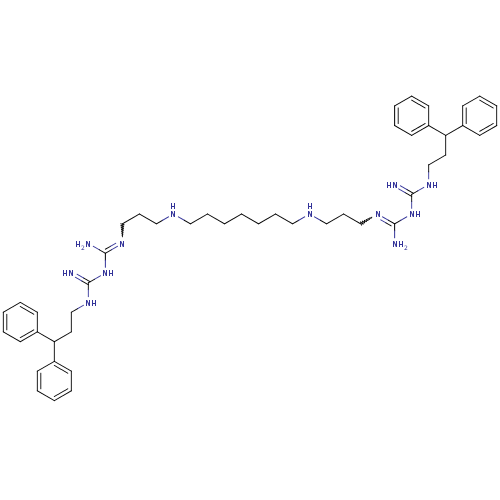
(CHEMBL378900 | N-(3,3-diphenylpropyl)-1-3-[3-({7-[...)Show SMILES NC(NC(=N)NCCC(c1ccccc1)c1ccccc1)=NCCCNCCCCCCCNCCCN=C(N)NC(=N)NCCC(c1ccccc1)c1ccccc1 |w:21.23,37.38| Show InChI InChI=1S/C47H68N12/c48-44(58-46(50)56-36-28-42(38-20-8-4-9-21-38)39-22-10-5-11-23-39)54-34-18-32-52-30-16-2-1-3-17-31-53-33-19-35-55-45(49)59-47(51)57-37-29-43(40-24-12-6-13-25-40)41-26-14-7-15-27-41/h4-15,20-27,42-43,52-53H,1-3,16-19,28-37H2,(H5,48,50,54,56,58)(H5,49,51,55,57,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50361473
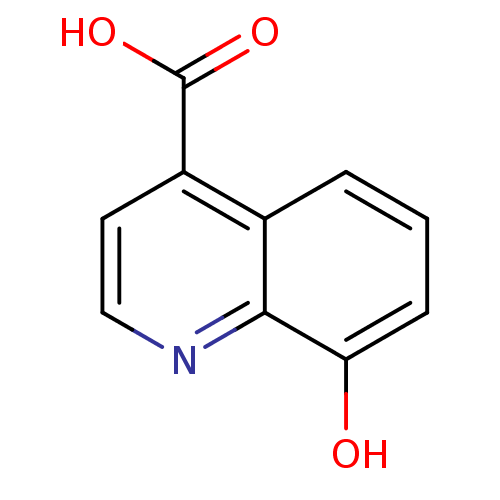
(CHEMBL1435274)Show InChI InChI=1S/C10H7NO3/c12-8-3-1-2-6-7(10(13)14)4-5-11-9(6)8/h1-5,12H,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM26106
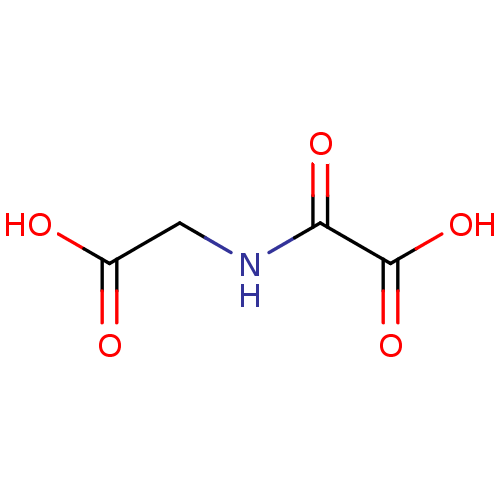
(CHEMBL90852 | N-oxalyl glycine, 1a | NOG | Oxalylg...)Show InChI InChI=1S/C4H5NO5/c6-2(7)1-5-3(8)4(9)10/h1H2,(H,5,8)(H,6,7)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50324535

(3-{[9-(Dimethylamino)nonanoyl](hydroxy)amino}propa...)Show InChI InChI=1S/C14H28N2O4/c1-15(2)11-8-6-4-3-5-7-9-13(17)16(20)12-10-14(18)19/h20H,3-12H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50361476
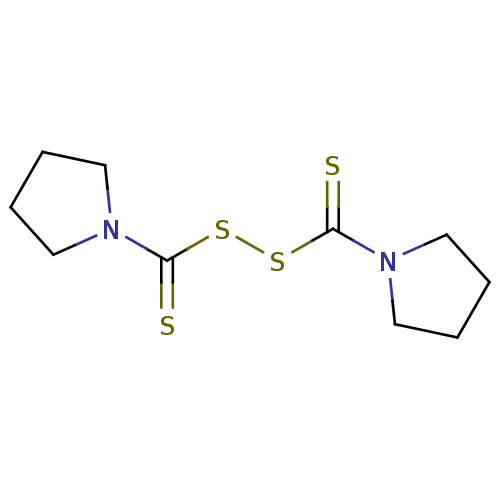
(CHEMBL583959)Show InChI InChI=1S/C10H16N2S4/c13-9(11-5-1-2-6-11)15-16-10(14)12-7-3-4-8-12/h1-8H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4C
(Homo sapiens (Human)) | BDBM50361475
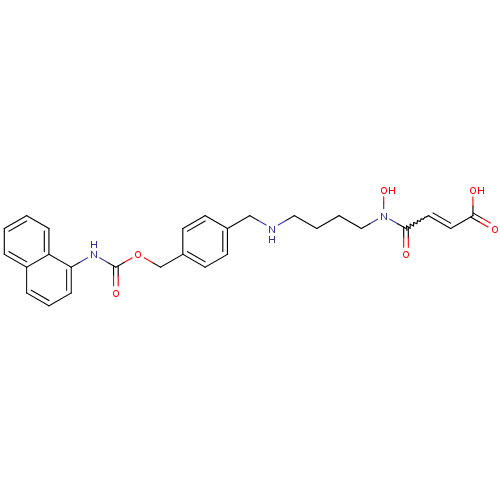
(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4C |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361470
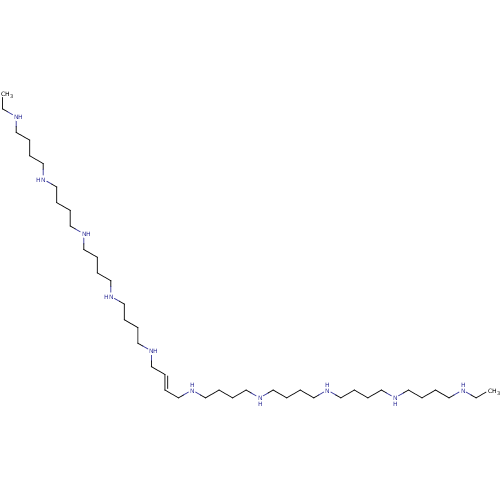
(CHEMBL226102)Show SMILES CCNCCCCNCCCCNCCCCNCCCCNC\C=C\CNCCCCNCCCCNCCCCNCCCCNCC Show InChI InChI=1S/C40H90N10/c1-3-41-23-5-7-25-43-27-9-11-29-45-31-13-15-33-47-35-17-19-37-49-39-21-22-40-50-38-20-18-36-48-34-16-14-32-46-30-12-10-28-44-26-8-6-24-42-4-2/h21-22,41-50H,3-20,23-40H2,1-2H3/b22-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM26106

(CHEMBL90852 | N-oxalyl glycine, 1a | NOG | Oxalylg...)Show InChI InChI=1S/C4H5NO5/c6-2(7)1-5-3(8)4(9)10/h1H2,(H,5,8)(H,6,7)(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone lysine demethylase PHF8
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM7B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50306580
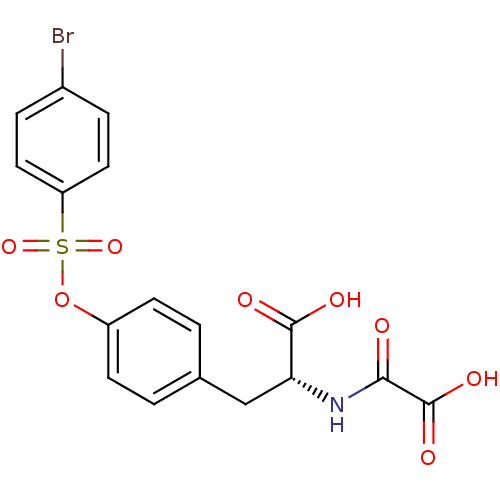
((R)-3-(4-(4-bromophenylsulfonyloxy)phenyl)-2-(carb...)Show SMILES OC(=O)[C@@H](Cc1ccc(OS(=O)(=O)c2ccc(Br)cc2)cc1)NC(=O)C(O)=O |r| Show InChI InChI=1S/C17H14BrNO8S/c18-11-3-7-13(8-4-11)28(25,26)27-12-5-1-10(2-6-12)9-14(16(21)22)19-15(20)17(23)24/h1-8,14H,9H2,(H,19,20)(H,21,22)(H,23,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50361473

(CHEMBL1435274)Show InChI InChI=1S/C10H7NO3/c12-8-3-1-2-6-7(10(13)14)4-5-11-9(6)8/h1-5,12H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha inhibitor
(Homo sapiens (Human)) | BDBM50361473

(CHEMBL1435274)Show InChI InChI=1S/C10H7NO3/c12-8-3-1-2-6-7(10(13)14)4-5-11-9(6)8/h1-5,12H,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of FIH |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha inhibitor
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of FIH |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50361471
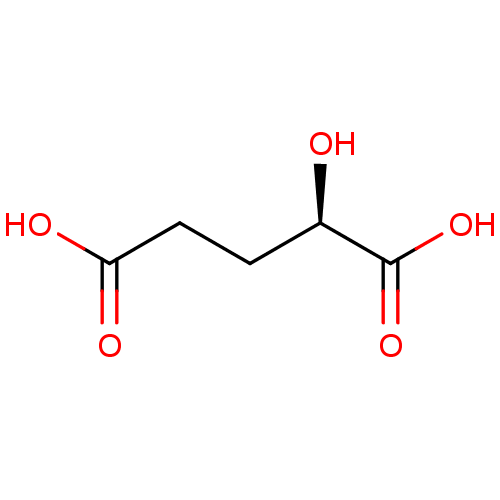
(CHEMBL1614745)Show InChI InChI=1S/C5H8O5/c6-3(5(9)10)1-2-4(7)8/h3,6H,1-2H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 3A
(Homo sapiens (Human)) | BDBM50361477
(CHEMBL56175) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM3A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50344962
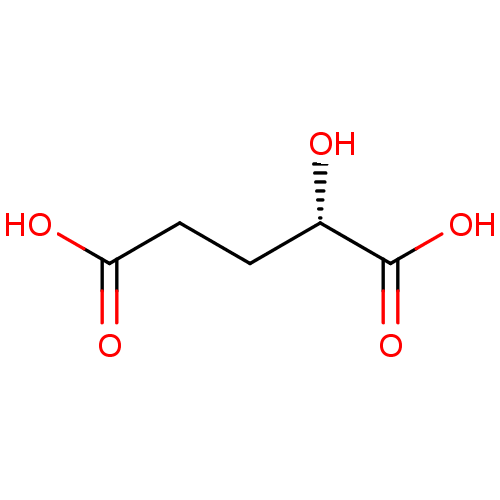
((2S)-2-HYDROXYPENTANEDIOIC ACID | CHEMBL1615211 | ...)Show InChI InChI=1S/C5H8O5/c6-3(5(9)10)1-2-4(7)8/h3,6H,1-2H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM4A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl hydroxylase EGLN3
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PHD3 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 6B
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM6B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50344962

((2S)-2-HYDROXYPENTANEDIOIC ACID | CHEMBL1615211 | ...)Show InChI InChI=1S/C5H8O5/c6-3(5(9)10)1-2-4(7)8/h3,6H,1-2H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of KDM2A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data