 Found 39 hits Enz. Inhib. hit(s) with all data for entry = 50034570
Found 39 hits Enz. Inhib. hit(s) with all data for entry = 50034570 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364407
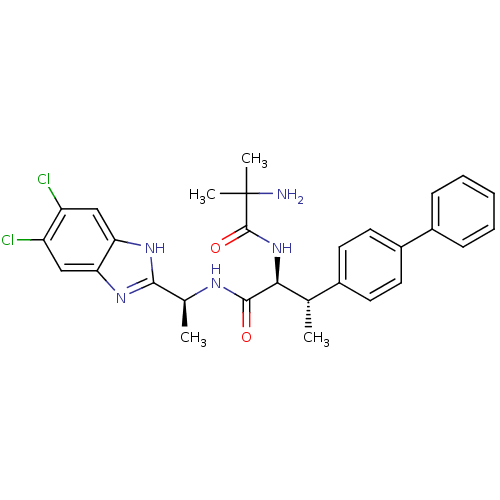
(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364386
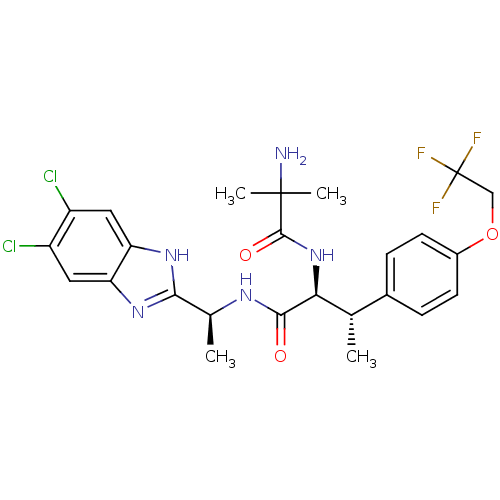
(CHEMBL1950440)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H28Cl2F3N5O3/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(35-23(37)24(3,4)31)22(36)32-13(2)21-33-18-9-16(26)17(27)10-19(18)34-21/h5-10,12-13,20H,11,31H2,1-4H3,(H,32,36)(H,33,34)(H,35,37)/t12-,13-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364407

(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364387
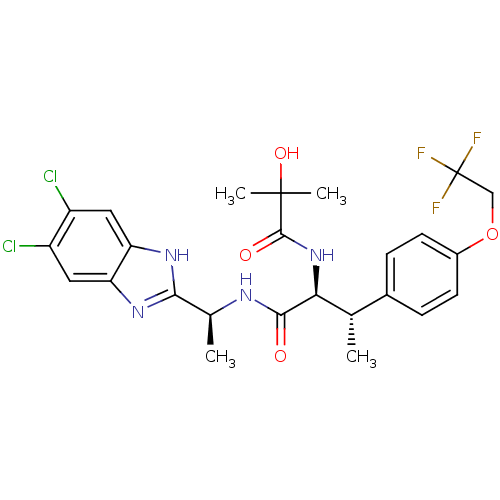
(CHEMBL1950439)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)O)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H27Cl2F3N4O4/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(34-23(36)24(3,4)37)22(35)31-13(2)21-32-18-9-16(26)17(27)10-19(18)33-21/h5-10,12-13,20,37H,11H2,1-4H3,(H,31,35)(H,32,33)(H,34,36)/t12-,13-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364408

(CHEMBL1950443)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NCC(C)(C)N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C28H31Cl2N5O/c1-17(26-34-23-14-21(29)22(30)15-24(23)35-26)33-27(36)25(32-16-28(2,3)31)13-18-9-11-20(12-10-18)19-7-5-4-6-8-19/h4-12,14-15,17,25,32H,13,16,31H2,1-3H3,(H,33,36)(H,34,35)/t17-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364406

(CHEMBL1950445)Show SMILES C[C@H](NC(=O)[C@@H](N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H24Cl2N4O/c1-14(16-8-10-18(11-9-16)17-6-4-3-5-7-17)23(28)25(32)29-15(2)24-30-21-12-19(26)20(27)13-22(21)31-24/h3-15,23H,28H2,1-2H3,(H,29,32)(H,30,31)/t14-,15-,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364406

(CHEMBL1950445)Show SMILES C[C@H](NC(=O)[C@@H](N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H24Cl2N4O/c1-14(16-8-10-18(11-9-16)17-6-4-3-5-7-17)23(28)25(32)29-15(2)24-30-21-12-19(26)20(27)13-22(21)31-24/h3-15,23H,28H2,1-2H3,(H,29,32)(H,30,31)/t14-,15-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364393
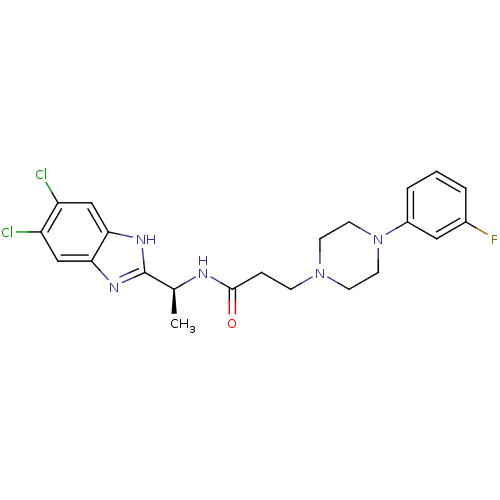
(CHEMBL1950309)Show SMILES C[C@H](NC(=O)CCN1CCN(CC1)c1cccc(F)c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C22H24Cl2FN5O/c1-14(22-27-19-12-17(23)18(24)13-20(19)28-22)26-21(31)5-6-29-7-9-30(10-8-29)16-4-2-3-15(25)11-16/h2-4,11-14H,5-10H2,1H3,(H,26,31)(H,27,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364388

(CHEMBL1950314)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)O)[C@@H](C)c1ccc(cc1)-c1cn[nH]c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C26H28Cl2N6O3/c1-13(15-5-7-16(8-6-15)17-11-29-30-12-17)22(34-25(36)26(3,4)37)24(35)31-14(2)23-32-20-9-18(27)19(28)10-21(20)33-23/h5-14,22,37H,1-4H3,(H,29,30)(H,31,35)(H,32,33)(H,34,36)/t13-,14-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364385

(CHEMBL1950441)Show SMILES C[C@H](NC(=O)[C@H](Cc1cccc(c1)C#N)NC(=O)C(C)(C)N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C23H24Cl2N6O2/c1-12(20-29-17-9-15(24)16(25)10-18(17)30-20)28-21(32)19(31-22(33)23(2,3)27)8-13-5-4-6-14(7-13)11-26/h4-7,9-10,12,19H,8,27H2,1-3H3,(H,28,32)(H,29,30)(H,31,33)/t12-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364395

(CHEMBL1950307)Show SMILES C[C@H](NC(=O)CCN1CCC(CC1)c1cccc(F)c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C23H25Cl2FN4O/c1-14(23-28-20-12-18(24)19(25)13-21(20)29-23)27-22(31)7-10-30-8-5-15(6-9-30)16-3-2-4-17(26)11-16/h2-4,11-15H,5-10H2,1H3,(H,27,31)(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364391
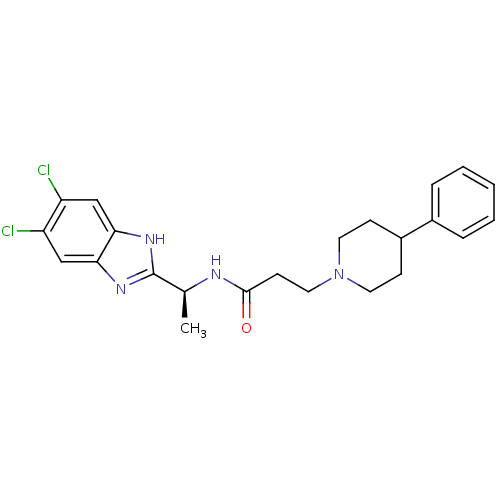
(CHEMBL1950311)Show SMILES C[C@H](NC(=O)CCN1CCC(CC1)c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C23H26Cl2N4O/c1-15(23-27-20-13-18(24)19(25)14-21(20)28-23)26-22(30)9-12-29-10-7-17(8-11-29)16-5-3-2-4-6-16/h2-6,13-15,17H,7-12H2,1H3,(H,26,30)(H,27,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364394

(CHEMBL1950310)Show SMILES C[C@H](NC(=O)CCN1CCN(CC1)c1ccc(F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C22H24Cl2FN5O/c1-14(22-27-19-12-17(23)18(24)13-20(19)28-22)26-21(31)6-7-29-8-10-30(11-9-29)16-4-2-15(25)3-5-16/h2-5,12-14H,6-11H2,1H3,(H,26,31)(H,27,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364407

(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328523
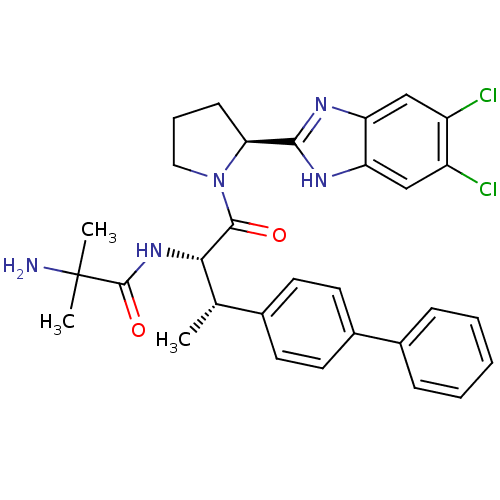
(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364392

(CHEMBL1950308)Show SMILES C[C@H](NC(=O)CCN1CCN(CC1)c1ccccc1F)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C22H24Cl2FN5O/c1-14(22-27-18-12-15(23)16(24)13-19(18)28-22)26-21(31)6-7-29-8-10-30(11-9-29)20-5-3-2-4-17(20)25/h2-5,12-14H,6-11H2,1H3,(H,26,31)(H,27,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364387

(CHEMBL1950439)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)O)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H27Cl2F3N4O4/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(34-23(36)24(3,4)37)22(35)31-13(2)21-32-18-9-16(26)17(27)10-19(18)33-21/h5-10,12-13,20,37H,11H2,1-4H3,(H,31,35)(H,32,33)(H,34,36)/t12-,13-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364403

(CHEMBL1950448)Show SMILES C[C@H](NC(=O)CCc1ccc(cc1)-c1cccnc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C23H20Cl2N4O/c1-14(23-28-20-11-18(24)19(25)12-21(20)29-23)27-22(30)9-6-15-4-7-16(8-5-15)17-3-2-10-26-13-17/h2-5,7-8,10-14H,6,9H2,1H3,(H,27,30)(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364400
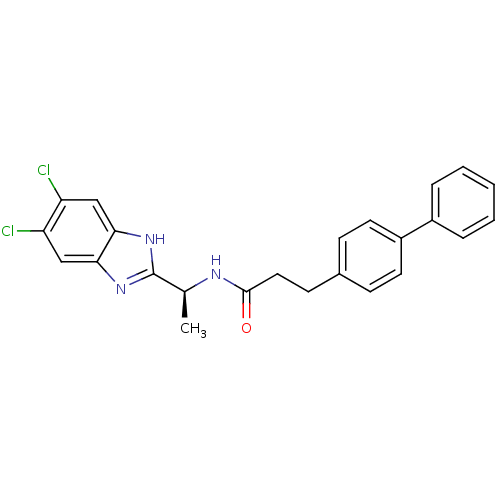
(CHEMBL1950451)Show SMILES C[C@H](NC(=O)CCc1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C24H21Cl2N3O/c1-15(24-28-21-13-19(25)20(26)14-22(21)29-24)27-23(30)12-9-16-7-10-18(11-8-16)17-5-3-2-4-6-17/h2-8,10-11,13-15H,9,12H2,1H3,(H,27,30)(H,28,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364388

(CHEMBL1950314)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)O)[C@@H](C)c1ccc(cc1)-c1cn[nH]c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C26H28Cl2N6O3/c1-13(15-5-7-16(8-6-15)17-11-29-30-12-17)22(34-25(36)26(3,4)37)24(35)31-14(2)23-32-20-9-18(27)19(28)10-21(20)33-23/h5-14,22,37H,1-4H3,(H,29,30)(H,31,35)(H,32,33)(H,34,36)/t13-,14-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364386

(CHEMBL1950440)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H28Cl2F3N5O3/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(35-23(37)24(3,4)31)22(36)32-13(2)21-33-18-9-16(26)17(27)10-19(18)34-21/h5-10,12-13,20H,11,31H2,1-4H3,(H,32,36)(H,33,34)(H,35,37)/t12-,13-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364409

(CHEMBL1950442)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1cccc(c1)C#N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C19H17Cl2N5O/c1-10(18-25-16-7-13(20)14(21)8-17(16)26-18)24-19(27)15(23)6-11-3-2-4-12(5-11)9-22/h2-5,7-8,10,15H,6,23H2,1H3,(H,24,27)(H,25,26)/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364385

(CHEMBL1950441)Show SMILES C[C@H](NC(=O)[C@H](Cc1cccc(c1)C#N)NC(=O)C(C)(C)N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C23H24Cl2N6O2/c1-12(20-29-17-9-15(24)16(25)10-18(17)30-20)28-21(32)19(31-22(33)23(2,3)27)8-13-5-4-6-14(7-13)11-26/h4-7,9-10,12,19H,8,27H2,1-3H3,(H,28,32)(H,29,30)(H,31,33)/t12-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364405

(CHEMBL1950446)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C24H22Cl2N4O/c1-14(23-29-21-12-18(25)19(26)13-22(21)30-23)28-24(31)20(27)11-15-7-9-17(10-8-15)16-5-3-2-4-6-16/h2-10,12-14,20H,11,27H2,1H3,(H,28,31)(H,29,30)/t14-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364390

(CHEMBL1950312)Show SMILES CC(C)(NC(=O)CCN1CCC(CC1)c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 Show InChI InChI=1S/C24H28Cl2N4O/c1-24(2,23-27-20-14-18(25)19(26)15-21(20)28-23)29-22(31)10-13-30-11-8-17(9-12-30)16-6-4-3-5-7-16/h3-7,14-15,17H,8-13H2,1-2H3,(H,27,28)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364402
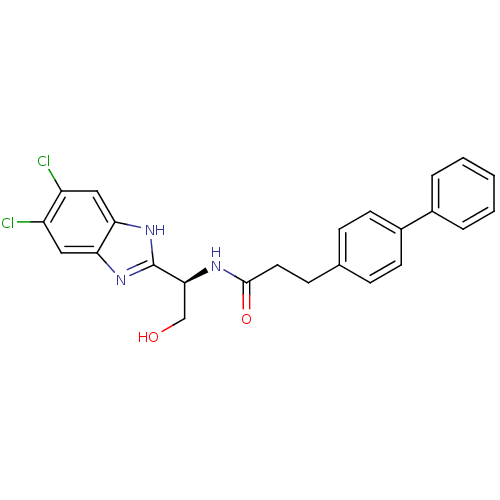
(CHEMBL1950449)Show SMILES OC[C@H](NC(=O)CCc1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C24H21Cl2N3O2/c25-18-12-20-21(13-19(18)26)29-24(28-20)22(14-30)27-23(31)11-8-15-6-9-17(10-7-15)16-4-2-1-3-5-16/h1-7,9-10,12-13,22,30H,8,11,14H2,(H,27,31)(H,28,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364401

(CHEMBL1950450)Show SMILES CC(C)(NC(=O)CCc1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 Show InChI InChI=1S/C25H23Cl2N3O/c1-25(2,24-28-21-14-19(26)20(27)15-22(21)29-24)30-23(31)13-10-16-8-11-18(12-9-16)17-6-4-3-5-7-17/h3-9,11-12,14-15H,10,13H2,1-2H3,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364389

(CHEMBL1950313)Show SMILES Clc1cc2nc(CNC(=O)CCN3CCC(CC3)c3ccccc3)[nH]c2cc1Cl Show InChI InChI=1S/C22H24Cl2N4O/c23-17-12-19-20(13-18(17)24)27-21(26-19)14-25-22(29)8-11-28-9-6-16(7-10-28)15-4-2-1-3-5-15/h1-5,12-13,16H,6-11,14H2,(H,25,29)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364408

(CHEMBL1950443)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NCC(C)(C)N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C28H31Cl2N5O/c1-17(26-34-23-14-21(29)22(30)15-24(23)35-26)33-27(36)25(32-16-28(2,3)31)13-18-9-11-20(12-10-18)19-7-5-4-6-8-19/h4-12,14-15,17,25,32H,13,16,31H2,1-3H3,(H,33,36)(H,34,35)/t17-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364406

(CHEMBL1950445)Show SMILES C[C@H](NC(=O)[C@@H](N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H24Cl2N4O/c1-14(16-8-10-18(11-9-16)17-6-4-3-5-7-17)23(28)25(32)29-15(2)24-30-21-12-19(26)20(27)13-22(21)31-24/h3-15,23H,28H2,1-2H3,(H,29,32)(H,30,31)/t14-,15-,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 638 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364404

(CHEMBL1950447)Show SMILES C[C@H](NC(=O)CCc1cccc(c1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C24H21Cl2N3O/c1-15(24-28-21-13-19(25)20(26)14-22(21)29-24)27-23(30)11-10-16-6-5-9-18(12-16)17-7-3-2-4-8-17/h2-9,12-15H,10-11H2,1H3,(H,27,30)(H,28,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 998 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364385

(CHEMBL1950441)Show SMILES C[C@H](NC(=O)[C@H](Cc1cccc(c1)C#N)NC(=O)C(C)(C)N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C23H24Cl2N6O2/c1-12(20-29-17-9-15(24)16(25)10-18(17)30-20)28-21(32)19(31-22(33)23(2,3)27)8-13-5-4-6-14(7-13)11-26/h4-7,9-10,12,19H,8,27H2,1-3H3,(H,28,32)(H,29,30)(H,31,33)/t12-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364393

(CHEMBL1950309)Show SMILES C[C@H](NC(=O)CCN1CCN(CC1)c1cccc(F)c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C22H24Cl2FN5O/c1-14(22-27-19-12-17(23)18(24)13-20(19)28-22)26-21(31)5-6-29-7-9-30(10-8-29)16-4-2-3-15(25)11-16/h2-4,11-14H,5-10H2,1H3,(H,26,31)(H,27,28)/t14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364409

(CHEMBL1950442)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1cccc(c1)C#N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C19H17Cl2N5O/c1-10(18-25-16-7-13(20)14(21)8-17(16)26-18)24-19(27)15(23)6-11-3-2-4-12(5-11)9-22/h2-5,7-8,10,15H,6,23H2,1H3,(H,24,27)(H,25,26)/t10-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364396

(CHEMBL1950455)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C32H34Cl2N4O3/c1-32(2,3)41-31(40)37-27(17-20-12-14-22(15-13-20)21-9-5-4-6-10-21)30(39)38-16-8-7-11-28(38)29-35-25-18-23(33)24(34)19-26(25)36-29/h4-6,9-10,12-15,18-19,27-28H,7-8,11,16-17H2,1-3H3,(H,35,36)(H,37,40)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364397
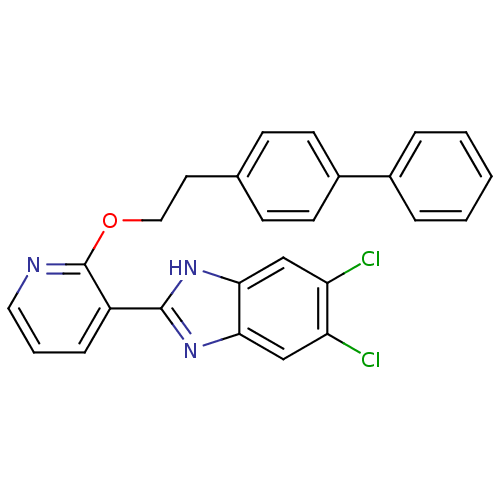
(CHEMBL1950454)Show SMILES Clc1cc2nc([nH]c2cc1Cl)-c1cccnc1OCCc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H19Cl2N3O/c27-21-15-23-24(16-22(21)28)31-25(30-23)20-7-4-13-29-26(20)32-14-12-17-8-10-19(11-9-17)18-5-2-1-3-6-18/h1-11,13,15-16H,12,14H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364398

(CHEMBL1950453)Show SMILES Clc1cc2nc([nH]c2cc1Cl)-c1cccnc1NCCc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H20Cl2N4/c27-21-15-23-24(16-22(21)28)32-26(31-23)20-7-4-13-29-25(20)30-14-12-17-8-10-19(11-9-17)18-5-2-1-3-6-18/h1-11,13,15-16H,12,14H2,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364399
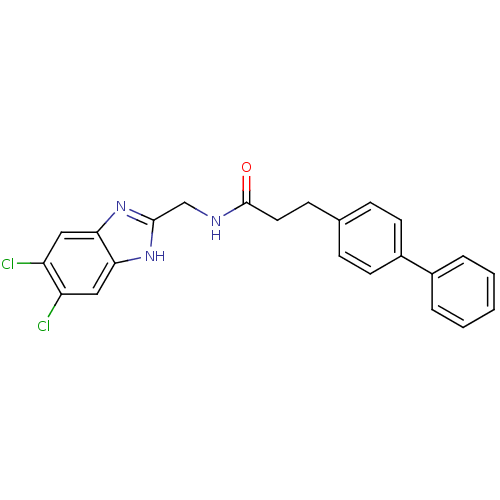
(CHEMBL1950452)Show SMILES Clc1cc2nc(CNC(=O)CCc3ccc(cc3)-c3ccccc3)[nH]c2cc1Cl Show InChI InChI=1S/C23H19Cl2N3O/c24-18-12-20-21(13-19(18)25)28-22(27-20)14-26-23(29)11-8-15-6-9-17(10-7-15)16-4-2-1-3-5-16/h1-7,9-10,12-13H,8,11,14H2,(H,26,29)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364394

(CHEMBL1950310)Show SMILES C[C@H](NC(=O)CCN1CCN(CC1)c1ccc(F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C22H24Cl2FN5O/c1-14(22-27-19-12-17(23)18(24)13-20(19)28-22)26-21(31)6-7-29-8-10-30(11-9-29)16-4-2-15(25)3-5-16/h2-5,12-14H,6-11H2,1H3,(H,26,31)(H,27,28)/t14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP-mediated angiotensin3 cleavage in mouse plasma using Mca-Ala-Lys-Dnp as substrate for 8 mins by LC/MS analysis |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data