 Found 32 hits Enz. Inhib. hit(s) with all data for entry = 50034631
Found 32 hits Enz. Inhib. hit(s) with all data for entry = 50034631 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of AXL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of cMET |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Jak3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of ABL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Src |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355489
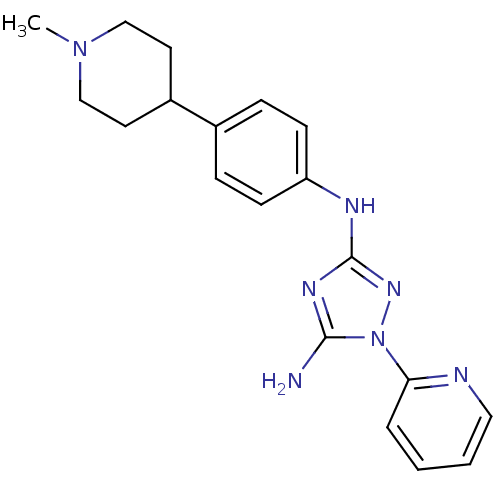
(CHEMBL1835867)Show SMILES CN1CCC(CC1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C19H23N7/c1-25-12-9-15(10-13-25)14-5-7-16(8-6-14)22-19-23-18(20)26(24-19)17-4-2-3-11-21-17/h2-8,11,15H,9-10,12-13H2,1H3,(H3,20,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of SCF-induced phosphorylation of c-KIT in human TF1 cells after 1 hr by immunoblot analysis |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT-mediated ERK 1/2 phosphorylation in human TF1 cells at 4 to 12 nM after 1 hr by immunoblot analysis |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355489

(CHEMBL1835867)Show SMILES CN1CCC(CC1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C19H23N7/c1-25-12-9-15(10-13-25)14-5-7-16(8-6-14)22-19-23-18(20)26(24-19)17-4-2-3-11-21-17/h2-8,11,15H,9-10,12-13H2,1H3,(H3,20,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814
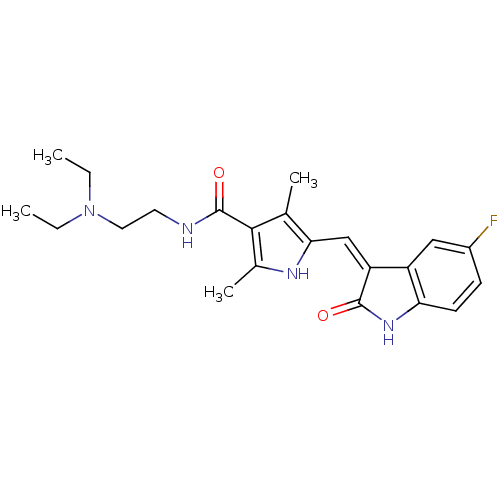
(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355479
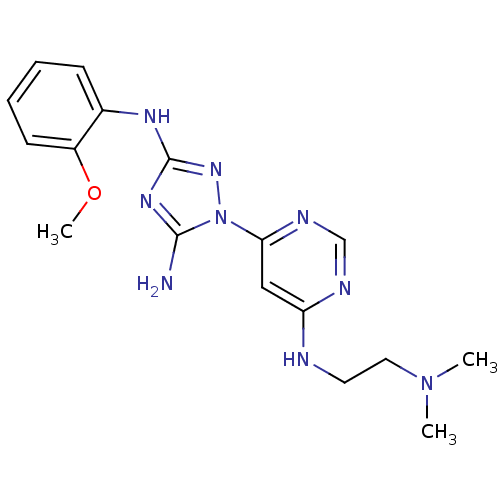
(CHEMBL1835746)Show InChI InChI=1S/C17H23N9O/c1-25(2)9-8-19-14-10-15(21-11-20-14)26-16(18)23-17(24-26)22-12-6-4-5-7-13(12)27-3/h4-7,10-11H,8-9H2,1-3H3,(H,19,20,21)(H3,18,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355480
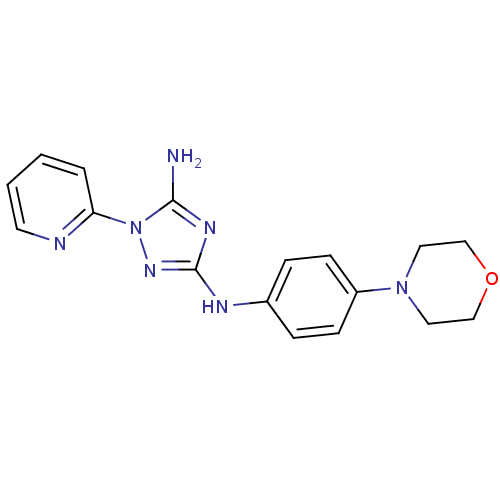
(CHEMBL1835747)Show InChI InChI=1S/C17H19N7O/c18-16-21-17(22-24(16)15-3-1-2-8-19-15)20-13-4-6-14(7-5-13)23-9-11-25-12-10-23/h1-8H,9-12H2,(H3,18,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355480

(CHEMBL1835747)Show InChI InChI=1S/C17H19N7O/c18-16-21-17(22-24(16)15-3-1-2-8-19-15)20-13-4-6-14(7-5-13)23-9-11-25-12-10-23/h1-8H,9-12H2,(H3,18,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355482
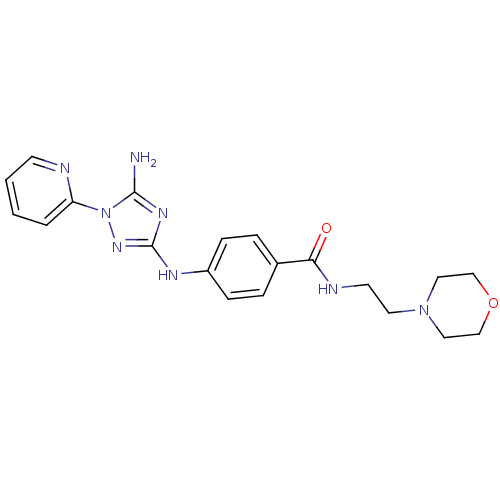
(CHEMBL1835749)Show SMILES Nc1nc(Nc2ccc(cc2)C(=O)NCCN2CCOCC2)nn1-c1ccccn1 Show InChI InChI=1S/C20H24N8O2/c21-19-25-20(26-28(19)17-3-1-2-8-22-17)24-16-6-4-15(5-7-16)18(29)23-9-10-27-11-13-30-14-12-27/h1-8H,9-14H2,(H,23,29)(H3,21,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355482

(CHEMBL1835749)Show SMILES Nc1nc(Nc2ccc(cc2)C(=O)NCCN2CCOCC2)nn1-c1ccccn1 Show InChI InChI=1S/C20H24N8O2/c21-19-25-20(26-28(19)17-3-1-2-8-22-17)24-16-6-4-15(5-7-16)18(29)23-9-10-27-11-13-30-14-12-27/h1-8H,9-14H2,(H,23,29)(H3,21,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355468
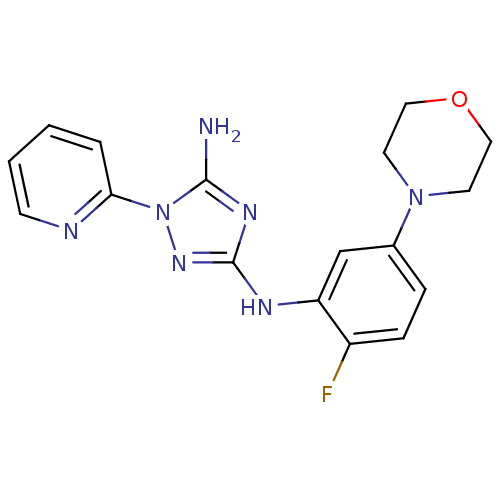
(CHEMBL1835743)Show InChI InChI=1S/C17H18FN7O/c18-13-5-4-12(24-7-9-26-10-8-24)11-14(13)21-17-22-16(19)25(23-17)15-3-1-2-6-20-15/h1-6,11H,7-10H2,(H3,19,21,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355467
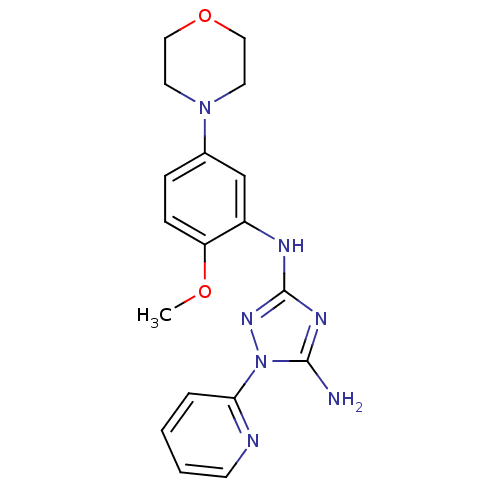
(CHEMBL1833992)Show InChI InChI=1S/C18H21N7O2/c1-26-15-6-5-13(24-8-10-27-11-9-24)12-14(15)21-18-22-17(19)25(23-18)16-4-2-3-7-20-16/h2-7,12H,8-11H2,1H3,(H3,19,21,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM13535
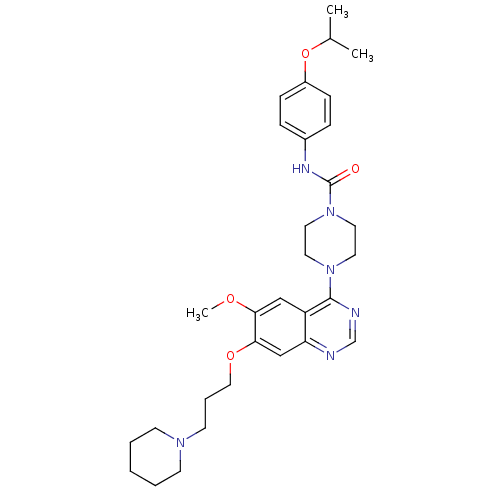
(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM13535

(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355468

(CHEMBL1835743)Show InChI InChI=1S/C17H18FN7O/c18-13-5-4-12(24-7-9-26-10-8-24)11-14(13)21-17-22-16(19)25(23-17)15-3-1-2-6-20-15/h1-6,11H,7-10H2,(H3,19,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355479

(CHEMBL1835746)Show InChI InChI=1S/C17H23N9O/c1-25(2)9-8-19-14-10-15(21-11-20-14)26-16(18)23-17(24-26)22-12-6-4-5-7-13(12)27-3/h4-7,10-11H,8-9H2,1-3H3,(H,19,20,21)(H3,18,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355467

(CHEMBL1833992)Show InChI InChI=1S/C18H21N7O2/c1-26-15-6-5-13(24-8-10-27-11-9-24)12-14(15)21-18-22-17(19)25(23-18)16-4-2-3-7-20-16/h2-7,12H,8-11H2,1H3,(H3,19,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Kit by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data