 Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50034927
Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50034927 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291683
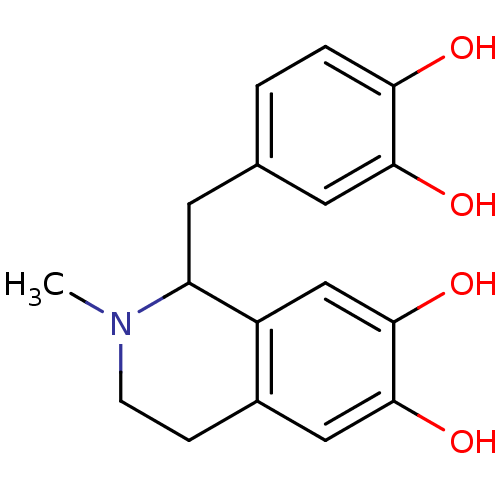
(1-(3,4-Dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C17H19NO4/c1-18-5-4-11-8-16(21)17(22)9-12(11)13(18)6-10-2-3-14(19)15(20)7-10/h2-3,7-9,13,19-22H,4-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681
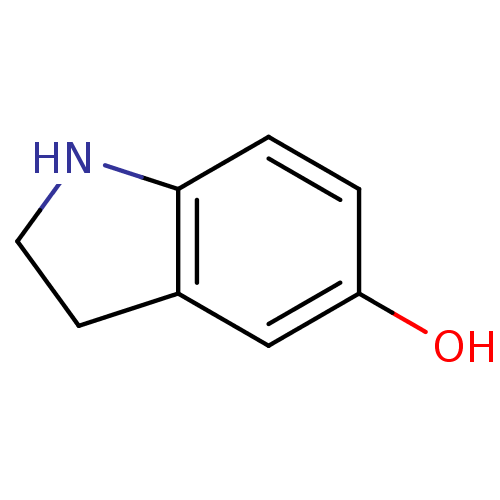
(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614
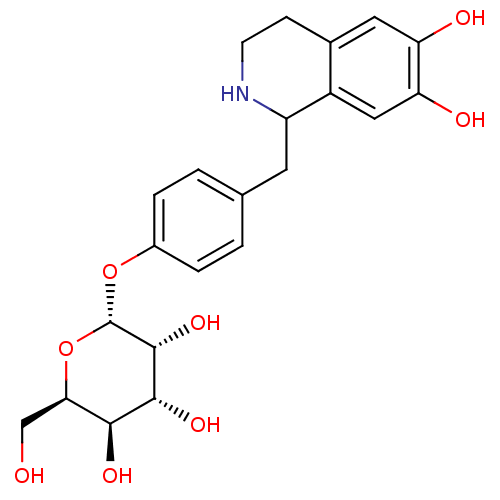
(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291683

(1-(3,4-Dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C17H19NO4/c1-18-5-4-11-8-16(21)17(22)9-12(11)13(18)6-10-2-3-14(19)15(20)7-10/h2-3,7-9,13,19-22H,4-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027331
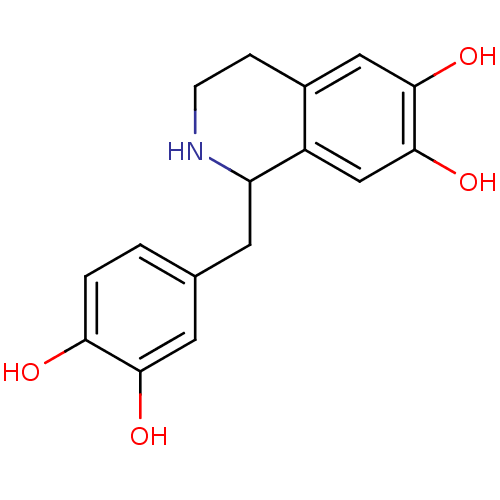
(1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquino...)Show InChI InChI=1S/C16H17NO4/c18-13-2-1-9(6-14(13)19)5-12-11-8-16(21)15(20)7-10(11)3-4-17-12/h1-2,6-8,12,17-21H,3-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50242856
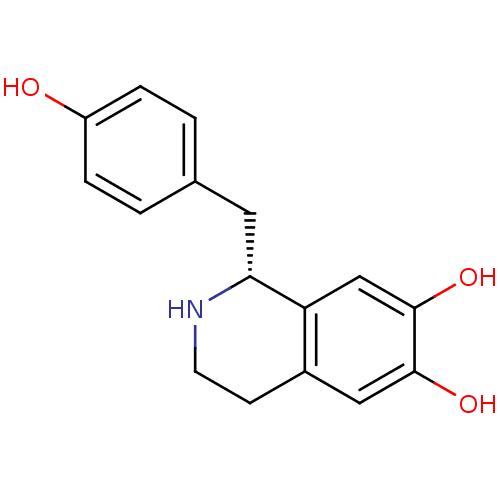
((1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquin...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)7-14-13-9-16(20)15(19)8-11(13)5-6-17-14/h1-4,8-9,14,17-20H,5-7H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027331

(1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquino...)Show InChI InChI=1S/C16H17NO4/c18-13-2-1-9(6-14(13)19)5-12-11-8-16(21)15(20)7-10(11)3-4-17-12/h1-2,6-8,12,17-21H,3-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50242856

((1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquin...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)7-14-13-9-16(20)15(19)8-11(13)5-6-17-14/h1-4,8-9,14,17-20H,5-7H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291684

(1-Methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol ...)Show InChI InChI=1S/C10H13NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-6,11-13H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291684

(1-Methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol ...)Show InChI InChI=1S/C10H13NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-6,11-13H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data