 Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50035321
Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50035321 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50131480
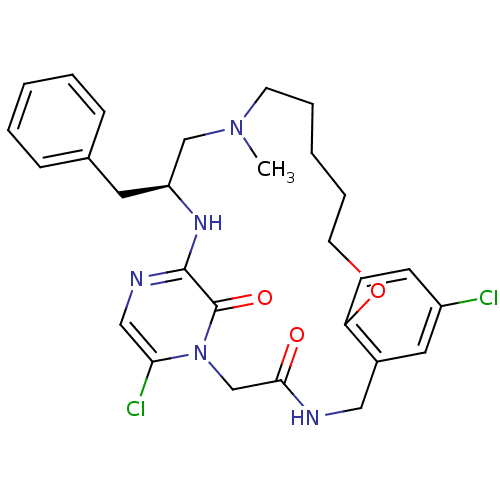
((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131460
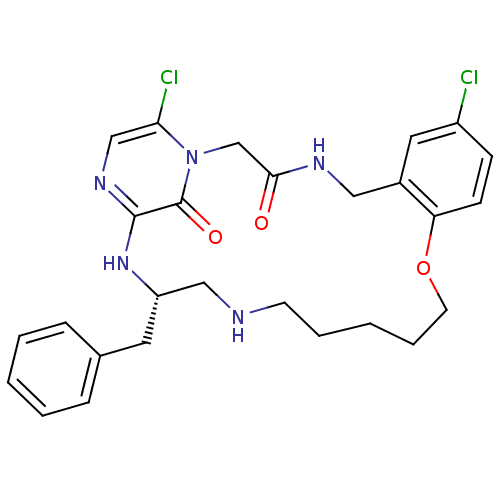
((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131464
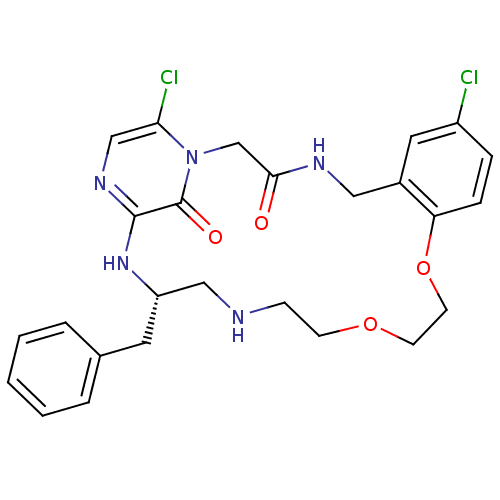
((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131471
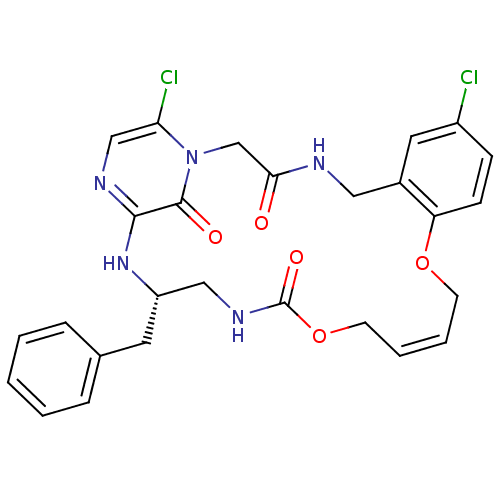
((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131470
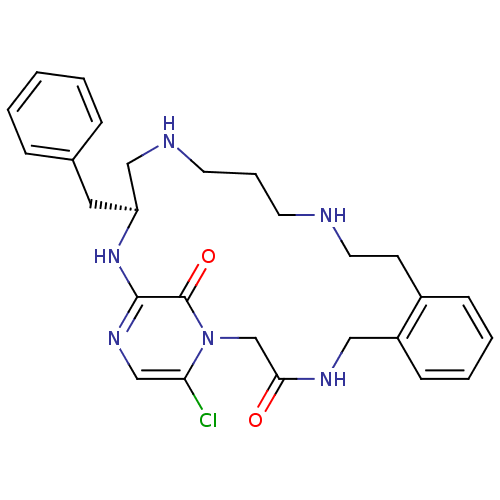
((11S)-11-BENZYL-6-CHLORO-1,2,10,11,12,13,14,15,16,...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCNCCc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H33ClN6O2/c28-24-18-32-26-27(36)34(24)19-25(35)31-16-22-10-5-4-9-21(22)11-14-29-12-6-13-30-17-23(33-26)15-20-7-2-1-3-8-20/h1-5,7-10,18,23,29-30H,6,11-17,19H2,(H,31,35)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50131473
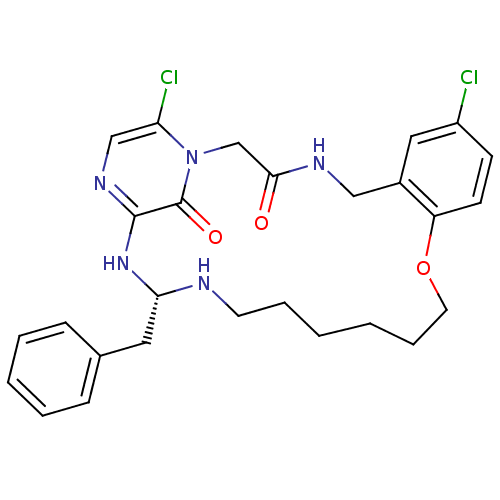
((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,19,21,23-pe...)Show SMILES Clc1ccc2OCCCCCCN[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-10-11-22-20(15-21)16-31-25(35)18-34-23(29)17-32-26(27(34)36)33-24(14-19-8-4-3-5-9-19)30-12-6-1-2-7-13-37-22/h3-5,8-11,15,17,24,30H,1-2,6-7,12-14,16,18H2,(H,31,35)(H,32,33)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131458
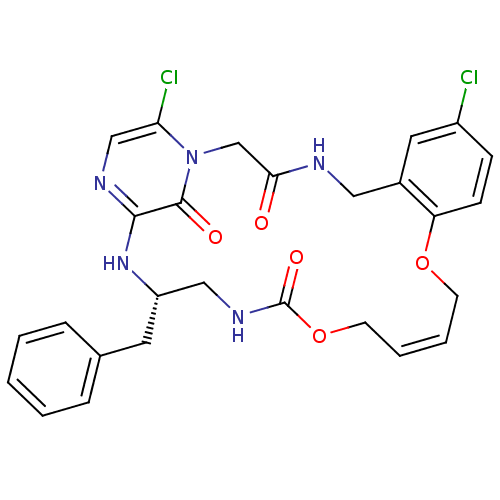
((14E,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C\COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |t:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4+/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131466
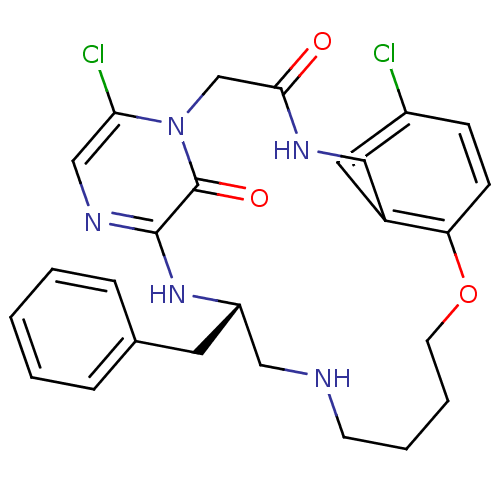
((S)-19-Benzyl-8,24-dichloro-12-oxa-1,4,17,20,22-pe...)Show SMILES Clc1ccc2OCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O3/c27-20-8-9-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-6-2-1-3-7-18)15-29-10-4-5-11-36-22/h1-3,6-9,13,16,21,29H,4-5,10-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131479
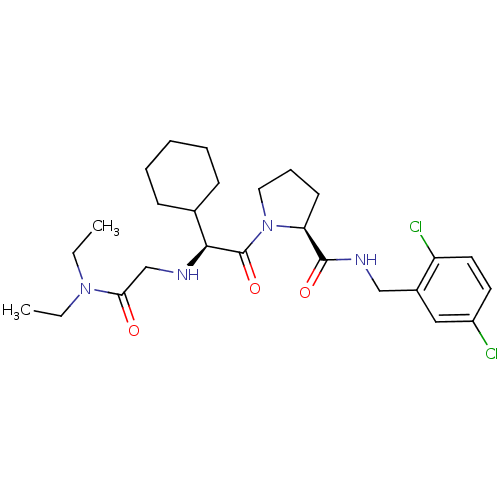
((S)-1-[(S)-3-Cyclohexyl-3-(diethylcarbamoylmethyl-...)Show SMILES CCN(CC)C(=O)CN[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1Cl Show InChI InChI=1S/C26H38Cl2N4O3/c1-3-31(4-2)23(33)17-29-24(18-9-6-5-7-10-18)26(35)32-14-8-11-22(32)25(34)30-16-19-15-20(27)12-13-21(19)28/h12-13,15,18,22,24,29H,3-11,14,16-17H2,1-2H3,(H,30,34)/t22-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131468

((S)-24-Chloro-11-(R)-cyclohexyl-20-oxa-3,9,12,17-t...)Show SMILES Clc1ccc2OCC(=O)NCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C26H35ClN4O5/c27-19-10-11-21-18(14-19)15-29-25(34)20-8-5-13-31(20)26(35)24(17-6-2-1-3-7-17)30-22(32)9-4-12-28-23(33)16-36-21/h10-11,14,17,20,24H,1-9,12-13,15-16H2,(H,28,33)(H,29,34)(H,30,32)/t20-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50056774
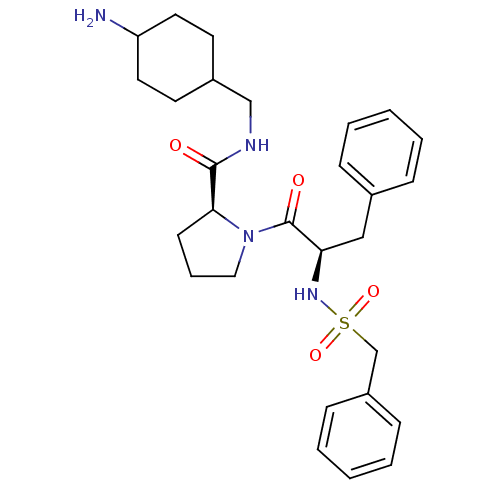
((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NS(=O)(=O)Cc2ccccc2)CC1 |wU:9.8,wD:16.25,(16.18,-5.42,;16.95,-6.75,;16.92,-8.41,;17.46,-9.87,;16.25,-10.69,;16.69,-12.16,;15.64,-13.28,;14.15,-12.93,;13.1,-14.05,;13.68,-11.46,;14.61,-10.23,;13.75,-8.97,;12.23,-9.43,;12.23,-10.97,;10.97,-11.84,;11.09,-13.38,;9.57,-11.18,;9.46,-9.66,;10.72,-8.77,;12.26,-8.77,;13,-7.44,;12.26,-6.1,;10.72,-6.1,;9.92,-7.44,;8.31,-12.07,;6.98,-12.83,;7.75,-14.19,;6.21,-11.49,;5.63,-13.61,;4.3,-12.84,;4.3,-11.3,;2.97,-10.53,;1.64,-11.3,;1.64,-12.86,;2.97,-13.61,;16.41,-9.22,;15.85,-7.85,)| Show InChI InChI=1S/C28H38N4O4S/c29-24-15-13-22(14-16-24)19-30-27(33)26-12-7-17-32(26)28(34)25(18-21-8-3-1-4-9-21)31-37(35,36)20-23-10-5-2-6-11-23/h1-6,8-11,22,24-26,31H,7,12-20,29H2,(H,30,33)/t22?,24?,25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131474

((S)-20-Benzyl-25-chloro-1,4,13,18,21,23-hexaaza-tr...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCCNCc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H33ClN6O2/c28-24-18-32-26-27(36)34(24)19-25(35)31-16-22-11-5-4-10-21(22)15-29-12-6-7-13-30-17-23(33-26)14-20-8-2-1-3-9-20/h1-5,8-11,18,23,29-30H,6-7,12-17,19H2,(H,31,35)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131465

((S)-21-Benzyl-8,26-dichloro-12,17-dioxa-1,4,19,22,...)Show SMILES Clc1ccc2OCCCCOC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H29Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-3,6-9,13,16,21H,4-5,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131475

(CHEMBL96893 | Ethyl-carbamic acid 2-({[(S)-1-((S)-...)Show SMILES CCNC(=O)Oc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C1CCCCC1 Show InChI InChI=1S/C23H33ClN4O4/c1-2-26-23(31)32-19-11-10-17(24)13-16(19)14-27-21(29)18-9-6-12-28(18)22(30)20(25)15-7-4-3-5-8-15/h10-11,13,15,18,20H,2-9,12,14,25H2,1H3,(H,26,31)(H,27,29)/t18-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131461
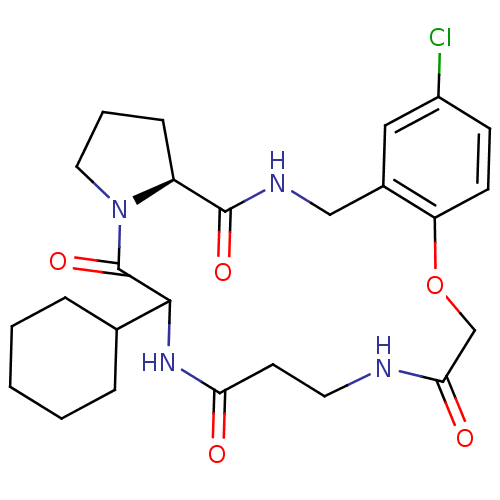
((S)-23-Chloro-11-cyclohexyl-19-oxa-3,9,12,16-tetra...)Show SMILES Clc1ccc2OCC(=O)NCCC(=O)NC(C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C25H33ClN4O5/c26-18-8-9-20-17(13-18)14-28-24(33)19-7-4-12-30(19)25(34)23(16-5-2-1-3-6-16)29-21(31)10-11-27-22(32)15-35-20/h8-9,13,16,19,23H,1-7,10-12,14-15H2,(H,27,32)(H,28,33)(H,29,31)/t19-,23?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131463
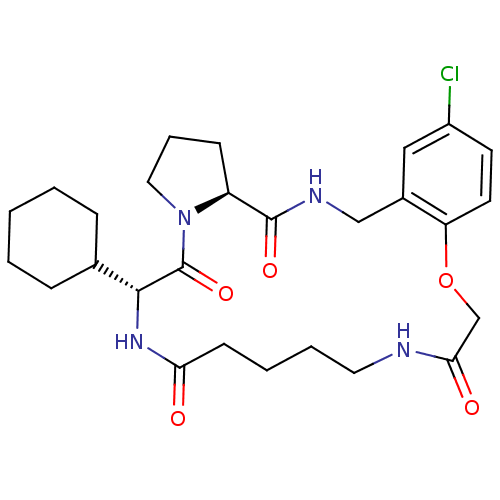
((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...)Show SMILES Clc1ccc2OCC(=O)NCCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C27H37ClN4O5/c28-20-11-12-22-19(15-20)16-30-26(35)21-9-6-14-32(21)27(36)25(18-7-2-1-3-8-18)31-23(33)10-4-5-13-29-24(34)17-37-22/h11-12,15,18,21,25H,1-10,13-14,16-17H2,(H,29,34)(H,30,35)(H,31,33)/t21-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131467
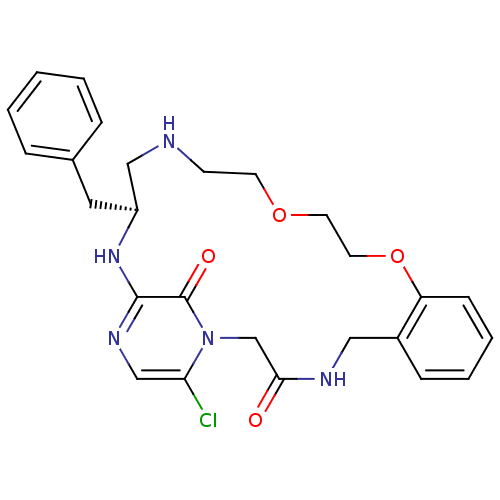
((S)-20-Benzyl-25-chloro-12,15-dioxa-1,4,18,21,23-p...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCOCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C26H30ClN5O4/c27-23-17-30-25-26(34)32(23)18-24(33)29-15-20-8-4-5-9-22(20)36-13-12-35-11-10-28-16-21(31-25)14-19-6-2-1-3-7-19/h1-9,17,21,28H,10-16,18H2,(H,29,33)(H,30,31)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131462
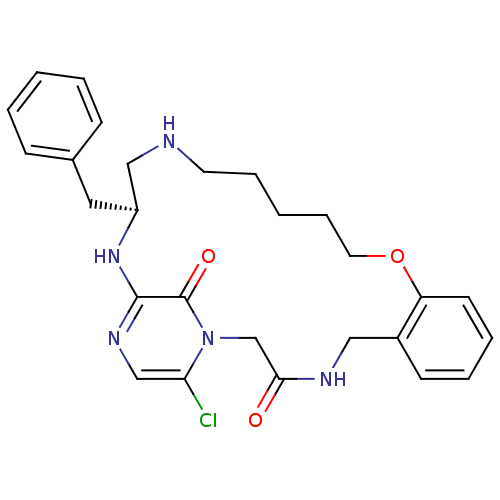
((S)-20-Benzyl-25-chloro-12-oxa-1,4,18,21,23-pentaa...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H32ClN5O3/c28-24-18-31-26-27(35)33(24)19-25(34)30-16-21-11-5-6-12-23(21)36-14-8-2-7-13-29-17-22(32-26)15-20-9-3-1-4-10-20/h1,3-6,9-12,18,22,29H,2,7-8,13-17,19H2,(H,30,34)(H,31,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131459

((S)-20-Benzyl-25-chloro-15-methyl-12-oxa-1,4,15,18...)Show SMILES CN1CCOc2ccccc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)CNC(=O)C1)c2=O Show InChI InChI=1S/C27H31ClN6O4/c1-33-11-12-38-22-10-6-5-9-20(22)14-29-25(36)18-34-23(28)16-31-26(27(34)37)32-21(15-30-24(35)17-33)13-19-7-3-2-4-8-19/h2-10,16,21H,11-15,17-18H2,1H3,(H,29,36)(H,30,35)(H,31,32)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131480

((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131476
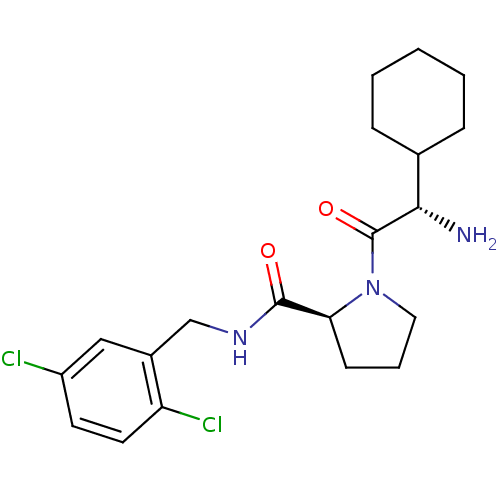
((S)-1-((S)-3-Amino-3-cyclohexyl-propionyl)-pyrroli...)Show SMILES N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1Cl Show InChI InChI=1S/C20H27Cl2N3O2/c21-15-8-9-16(22)14(11-15)12-24-19(26)17-7-4-10-25(17)20(27)18(23)13-5-2-1-3-6-13/h8-9,11,13,17-18H,1-7,10,12,23H2,(H,24,26)/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50366827
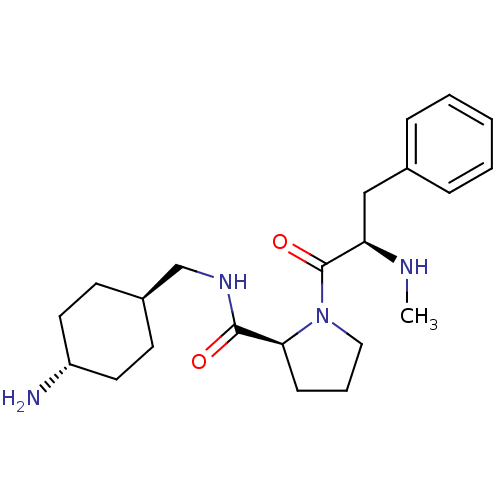
(CHEMBL125181 | L-371912)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC[C@H]1CC[C@H](N)CC1 |wU:16.18,21.22,wD:2.1,24.26,(.43,-4.06,;1.52,-5.16,;3.02,-4.76,;3.15,-3.22,;1.89,-2.34,;2.88,-1.15,;2.33,.28,;.82,.53,;-.16,-.66,;.38,-2.08,;4.42,-5.41,;4.55,-6.95,;5.68,-4.53,;5.69,-2.99,;7.17,-2.53,;8.05,-3.8,;7.12,-5.02,;7.89,-6.35,;7.87,-7.89,;9.38,-5.96,;10.46,-7.04,;11.78,-6.27,;13.32,-6.27,;14.89,-4.99,;16.21,-6.19,;17.54,-5.41,;14.77,-6.16,;13.11,-7.46,)| Show InChI InChI=1S/C22H34N4O2/c1-24-19(14-16-6-3-2-4-7-16)22(28)26-13-5-8-20(26)21(27)25-15-17-9-11-18(23)12-10-17/h2-4,6-7,17-20,24H,5,8-15,23H2,1H3,(H,25,27)/t17-,18-,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131460

((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131471

((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131466

((S)-19-Benzyl-8,24-dichloro-12-oxa-1,4,17,20,22-pe...)Show SMILES Clc1ccc2OCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O3/c27-20-8-9-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-6-2-1-3-7-18)15-29-10-4-5-11-36-22/h1-3,6-9,13,16,21,29H,4-5,10-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131478

((S)-18-Benzyl-8,23-dichloro-12-oxa-1,4,16,19,21-pe...)Show SMILES Clc1ccc2OCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C25H27Cl2N5O3/c26-19-7-8-21-18(12-19)13-29-23(33)16-32-22(27)15-30-24(25(32)34)31-20(14-28-9-4-10-35-21)11-17-5-2-1-3-6-17/h1-3,5-8,12,15,20,28H,4,9-11,13-14,16H2,(H,29,33)(H,30,31)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131480

((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131460

((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131466

((S)-19-Benzyl-8,24-dichloro-12-oxa-1,4,17,20,22-pe...)Show SMILES Clc1ccc2OCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O3/c27-20-8-9-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-6-2-1-3-7-18)15-29-10-4-5-11-36-22/h1-3,6-9,13,16,21,29H,4-5,10-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131471

((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131464

((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131464

((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131473

((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,19,21,23-pe...)Show SMILES Clc1ccc2OCCCCCCN[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-10-11-22-20(15-21)16-31-25(35)18-34-23(29)17-32-26(27(34)36)33-24(14-19-8-4-3-5-9-19)30-12-6-1-2-7-13-37-22/h3-5,8-11,15,17,24,30H,1-2,6-7,12-14,16,18H2,(H,31,35)(H,32,33)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131462

((S)-20-Benzyl-25-chloro-12-oxa-1,4,18,21,23-pentaa...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H32ClN5O3/c28-24-18-31-26-27(35)33(24)19-25(34)30-16-21-11-5-6-12-23(21)36-14-8-2-7-13-29-17-22(32-26)15-20-9-3-1-4-10-20/h1,3-6,9-12,18,22,29H,2,7-8,13-17,19H2,(H,30,34)(H,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131458

((14E,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C\COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |t:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4+/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131472

((S)-17-Benzyl-8,22-dichloro-12-oxa-1,4,15,18,20-pe...)Show SMILES Clc1ccc2OCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C24H25Cl2N5O3/c25-18-6-7-20-17(11-18)12-28-22(32)15-31-21(26)14-29-23(24(31)33)30-19(13-27-8-9-34-20)10-16-4-2-1-3-5-16/h1-7,11,14,19,27H,8-10,12-13,15H2,(H,28,32)(H,29,30)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131458

((14E,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C\COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |t:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4+/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131470

((11S)-11-BENZYL-6-CHLORO-1,2,10,11,12,13,14,15,16,...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCNCCc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H33ClN6O2/c28-24-18-32-26-27(36)34(24)19-25(35)31-16-22-10-5-4-9-21(22)11-14-29-12-6-13-30-17-23(33-26)15-20-7-2-1-3-8-20/h1-5,7-10,18,23,29-30H,6,11-17,19H2,(H,31,35)(H,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131470

((11S)-11-BENZYL-6-CHLORO-1,2,10,11,12,13,14,15,16,...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCNCCc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H33ClN6O2/c28-24-18-32-26-27(36)34(24)19-25(35)31-16-22-10-5-4-9-21(22)11-14-29-12-6-13-30-17-23(33-26)15-20-7-2-1-3-8-20/h1-5,7-10,18,23,29-30H,6,11-17,19H2,(H,31,35)(H,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131467

((S)-20-Benzyl-25-chloro-12,15-dioxa-1,4,18,21,23-p...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCOCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C26H30ClN5O4/c27-23-17-30-25-26(34)32(23)18-24(33)29-15-20-8-4-5-9-22(20)36-13-12-35-11-10-28-16-21(31-25)14-19-6-2-1-3-7-19/h1-9,17,21,28H,10-16,18H2,(H,29,33)(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131459

((S)-20-Benzyl-25-chloro-15-methyl-12-oxa-1,4,15,18...)Show SMILES CN1CCOc2ccccc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)CNC(=O)C1)c2=O Show InChI InChI=1S/C27H31ClN6O4/c1-33-11-12-38-22-10-6-5-9-20(22)14-29-25(36)18-34-23(28)16-31-26(27(34)37)32-21(15-30-24(35)17-33)13-19-7-3-2-4-8-19/h2-10,16,21H,11-15,17-18H2,1H3,(H,29,36)(H,30,35)(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131465

((S)-21-Benzyl-8,26-dichloro-12,17-dioxa-1,4,19,22,...)Show SMILES Clc1ccc2OCCCCOC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H29Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-3,6-9,13,16,21H,4-5,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131463

((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...)Show SMILES Clc1ccc2OCC(=O)NCCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C27H37ClN4O5/c28-20-11-12-22-19(15-20)16-30-26(35)21-9-6-14-32(21)27(36)25(18-7-2-1-3-8-18)31-23(33)10-4-5-13-29-24(34)17-37-22/h11-12,15,18,21,25H,1-10,13-14,16-17H2,(H,29,34)(H,30,35)(H,31,33)/t21-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131467

((S)-20-Benzyl-25-chloro-12,15-dioxa-1,4,18,21,23-p...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCOCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C26H30ClN5O4/c27-23-17-30-25-26(34)32(23)18-24(33)29-15-20-8-4-5-9-22(20)36-13-12-35-11-10-28-16-21(31-25)14-19-6-2-1-3-7-19/h1-9,17,21,28H,10-16,18H2,(H,29,33)(H,30,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131468

((S)-24-Chloro-11-(R)-cyclohexyl-20-oxa-3,9,12,17-t...)Show SMILES Clc1ccc2OCC(=O)NCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C26H35ClN4O5/c27-19-10-11-21-18(14-19)15-29-25(34)20-8-5-13-31(20)26(35)24(17-6-2-1-3-7-17)30-22(32)9-4-12-28-23(33)16-36-21/h10-11,14,17,20,24H,1-9,12-13,15-16H2,(H,28,33)(H,29,34)(H,30,32)/t20-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131461

((S)-23-Chloro-11-cyclohexyl-19-oxa-3,9,12,16-tetra...)Show SMILES Clc1ccc2OCC(=O)NCCC(=O)NC(C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C25H33ClN4O5/c26-18-8-9-20-17(13-18)14-28-24(33)19-7-4-12-30(19)25(34)23(16-5-2-1-3-6-16)29-21(31)10-11-27-22(32)15-35-20/h8-9,13,16,19,23H,1-7,10-12,14-15H2,(H,27,32)(H,28,33)(H,29,31)/t19-,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131462

((S)-20-Benzyl-25-chloro-12-oxa-1,4,18,21,23-pentaa...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H32ClN5O3/c28-24-18-31-26-27(35)33(24)19-25(34)30-16-21-11-5-6-12-23(21)36-14-8-2-7-13-29-17-22(32-26)15-20-9-3-1-4-10-20/h1,3-6,9-12,18,22,29H,2,7-8,13-17,19H2,(H,30,34)(H,31,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131468

((S)-24-Chloro-11-(R)-cyclohexyl-20-oxa-3,9,12,17-t...)Show SMILES Clc1ccc2OCC(=O)NCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C26H35ClN4O5/c27-19-10-11-21-18(14-19)15-29-25(34)20-8-5-13-31(20)26(35)24(17-6-2-1-3-7-17)30-22(32)9-4-12-28-23(33)16-36-21/h10-11,14,17,20,24H,1-9,12-13,15-16H2,(H,28,33)(H,29,34)(H,30,32)/t20-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50131469

((S)-22-Chloro-11-cyclohexyl-18-oxa-3,9,12,15-tetra...)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](NC(=O)CNC(=O)COc1ccc(Cl)cc1CNC2=O)C1CCCCC1 |r| Show InChI InChI=1S/C24H31ClN4O5/c25-17-8-9-19-16(11-17)12-27-23(32)18-7-4-10-29(18)24(33)22(15-5-2-1-3-6-15)28-20(30)13-26-21(31)14-34-19/h8-9,11,15,18,22H,1-7,10,12-14H2,(H,26,31)(H,27,32)(H,28,30)/t18-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human trypsin |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131469

((S)-22-Chloro-11-cyclohexyl-18-oxa-3,9,12,15-tetra...)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](NC(=O)CNC(=O)COc1ccc(Cl)cc1CNC2=O)C1CCCCC1 |r| Show InChI InChI=1S/C24H31ClN4O5/c25-17-8-9-19-16(11-17)12-27-23(32)18-7-4-10-29(18)24(33)22(15-5-2-1-3-6-15)28-20(30)13-26-21(31)14-34-19/h8-9,11,15,18,22H,1-7,10,12-14H2,(H,26,31)(H,27,32)(H,28,30)/t18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data