 Found 68 hits Enz. Inhib. hit(s) with all data for entry = 50035408
Found 68 hits Enz. Inhib. hit(s) with all data for entry = 50035408 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10872
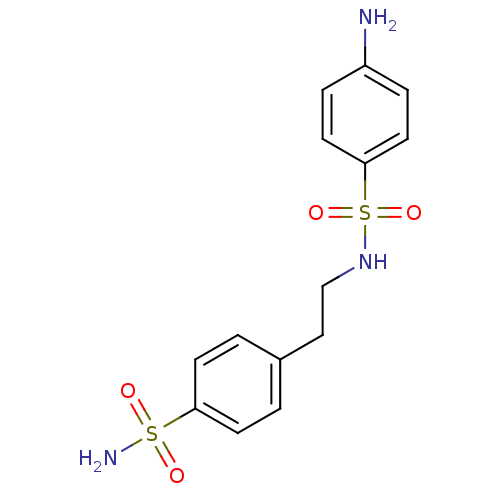
(4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...)Show SMILES Nc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H17N3O4S2/c15-12-3-7-14(8-4-12)23(20,21)17-10-9-11-1-5-13(6-2-11)22(16,18)19/h1-8,17H,9-10,15H2,(H2,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10871
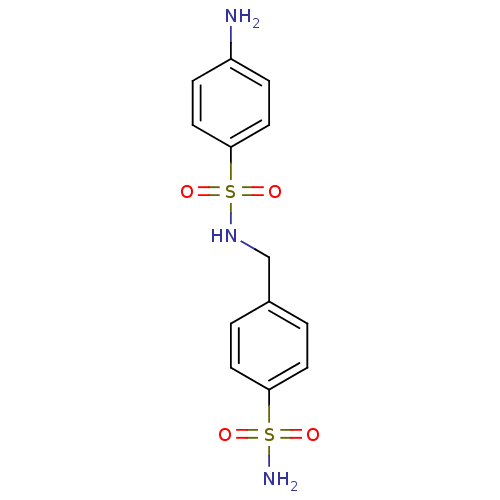
(4-amino-N-[(4-sulfamoylphenyl)methyl]benzene-1-sul...)Show InChI InChI=1S/C13H15N3O4S2/c14-11-3-7-13(8-4-11)22(19,20)16-9-10-1-5-12(6-2-10)21(15,17)18/h1-8,16H,9,14H2,(H2,15,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM50080733
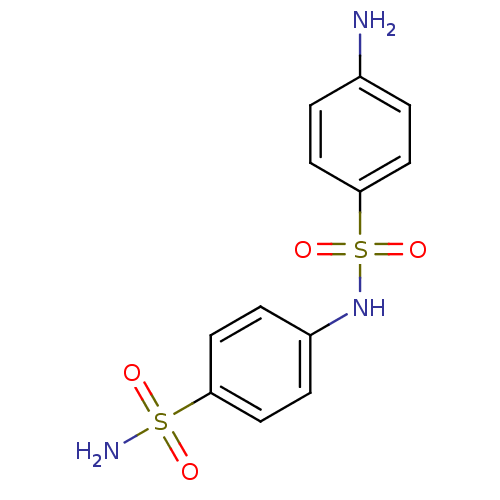
(4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-1-5-12(6-2-9)21(18,19)15-10-3-7-11(8-4-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (hCA I) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10872

(4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...)Show SMILES Nc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H17N3O4S2/c15-12-3-7-14(8-4-12)23(20,21)17-10-9-11-1-5-13(6-2-11)22(16,18)19/h1-8,17H,9-10,15H2,(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10872

(4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...)Show SMILES Nc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H17N3O4S2/c15-12-3-7-14(8-4-12)23(20,21)17-10-9-11-1-5-13(6-2-11)22(16,18)19/h1-8,17H,9-10,15H2,(H2,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10871

(4-amino-N-[(4-sulfamoylphenyl)methyl]benzene-1-sul...)Show InChI InChI=1S/C13H15N3O4S2/c14-11-3-7-13(8-4-11)22(19,20)16-9-10-1-5-12(6-2-10)21(15,17)18/h1-8,16H,9,14H2,(H2,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10857
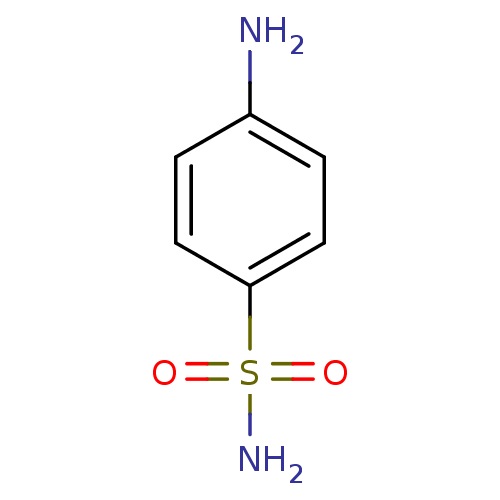
(4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...)Show InChI InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10861
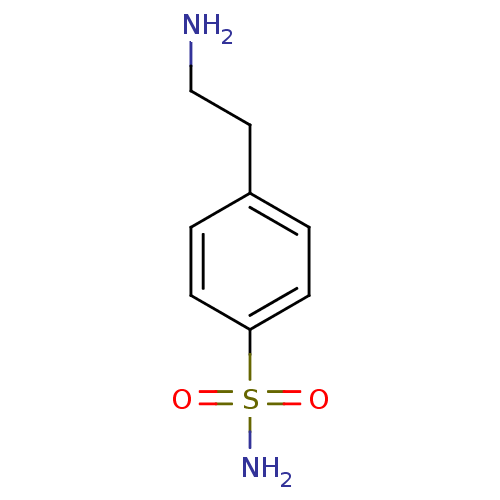
(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10871

(4-amino-N-[(4-sulfamoylphenyl)methyl]benzene-1-sul...)Show InChI InChI=1S/C13H15N3O4S2/c14-11-3-7-13(8-4-11)22(19,20)16-9-10-1-5-12(6-2-10)21(15,17)18/h1-8,16H,9,14H2,(H2,15,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10855
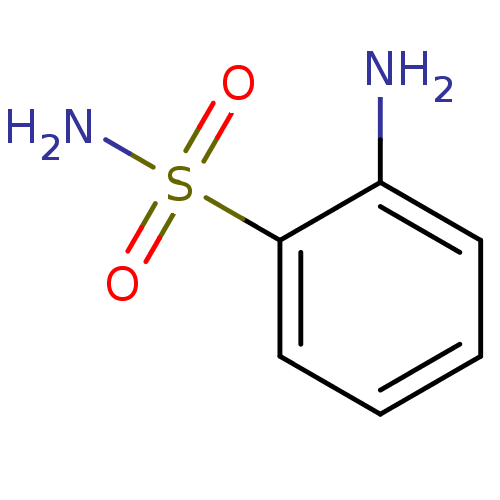
(2-aminobenzene-1-sulfonamide | CHEMBL6705 | US1017...)Show InChI InChI=1S/C6H8N2O2S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50080733

(4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-1-5-12(6-2-9)21(18,19)15-10-3-7-11(8-4-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10864
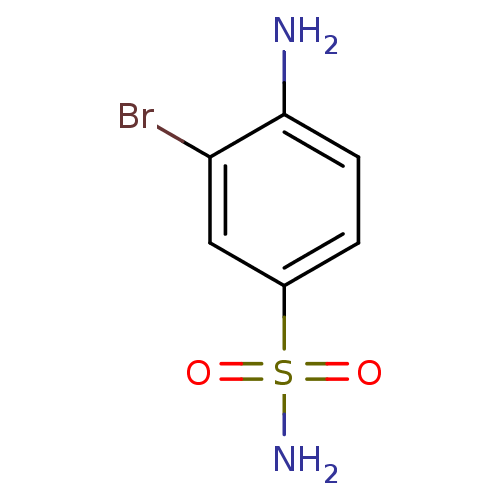
(4-Amino-3-bromobenzenesulfonamide | 4-amino-3-brom...)Show InChI InChI=1S/C6H7BrN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10860
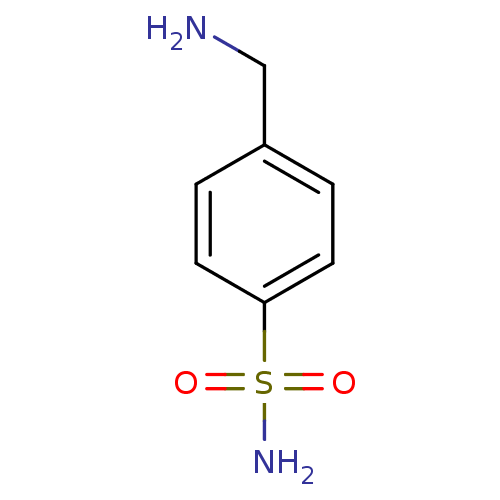
(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10855

(2-aminobenzene-1-sulfonamide | CHEMBL6705 | US1017...)Show InChI InChI=1S/C6H8N2O2S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10861

(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080733

(4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-1-5-12(6-2-9)21(18,19)15-10-3-7-11(8-4-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10865
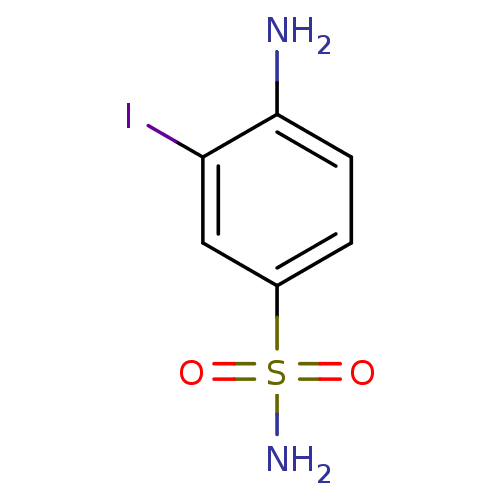
(4-Amino-3-iodobenzenesulfonamide | 4-amino-3-iodob...)Show InChI InChI=1S/C6H7IN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (hCA I) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10862
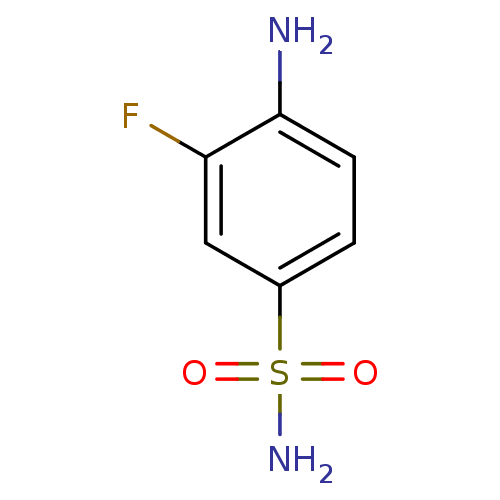
(4-Amino-3-fluorobenzenesulfonamide | 4-amino-3-flu...)Show InChI InChI=1S/C6H7FN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10864

(4-Amino-3-bromobenzenesulfonamide | 4-amino-3-brom...)Show InChI InChI=1S/C6H7BrN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM10863
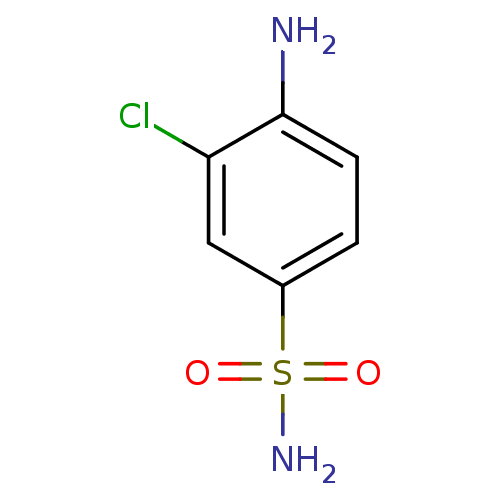
(4-Amino-3-chlorobenzenesulfonamide | 4-amino-3-chl...)Show InChI InChI=1S/C6H7ClN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine carbonic anhydrase XIII (mCA XIII) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10862

(4-Amino-3-fluorobenzenesulfonamide | 4-amino-3-flu...)Show InChI InChI=1S/C6H7FN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10865

(4-Amino-3-iodobenzenesulfonamide | 4-amino-3-iodob...)Show InChI InChI=1S/C6H7IN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10872

(4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...)Show SMILES Nc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H17N3O4S2/c15-12-3-7-14(8-4-12)23(20,21)17-10-9-11-1-5-13(6-2-11)22(16,18)19/h1-8,17H,9-10,15H2,(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (hCA I) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10871

(4-amino-N-[(4-sulfamoylphenyl)methyl]benzene-1-sul...)Show InChI InChI=1S/C13H15N3O4S2/c14-11-3-7-13(8-4-11)22(19,20)16-9-10-1-5-12(6-2-10)21(15,17)18/h1-8,16H,9,14H2,(H2,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (hCA I) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10863

(4-Amino-3-chlorobenzenesulfonamide | 4-amino-3-chl...)Show InChI InChI=1S/C6H7ClN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10861

(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50080733

(4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-1-5-12(6-2-9)21(18,19)15-10-3-7-11(8-4-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (hCA I) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (hCA II) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10862

(4-Amino-3-fluorobenzenesulfonamide | 4-amino-3-flu...)Show InChI InChI=1S/C6H7FN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (hCA I) by using esterase assay method |
Bioorg Med Chem Lett 14: 3757-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.106
BindingDB Entry DOI: 10.7270/Q24Q7VJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data