 Found 99 hits Enz. Inhib. hit(s) with all data for entry = 50049278
Found 99 hits Enz. Inhib. hit(s) with all data for entry = 50049278 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217933

(US9212182, 674)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16F3N3O5S/c1-35-23-12-17(14-2-5-18(26)20(28)11-14)19(27)13-22(23)31-21-6-4-16(10-15(21)3-7-25(31)32)37(33,34)30-24-8-9-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217798
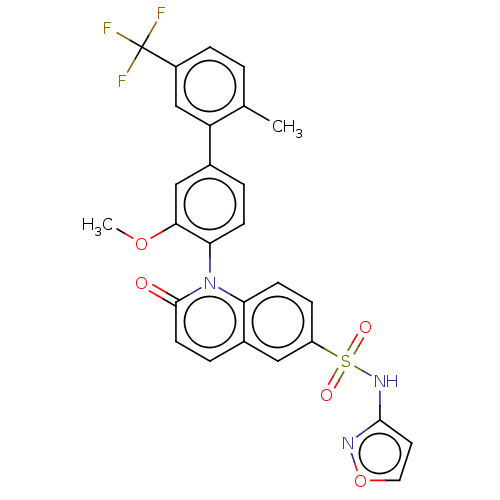
(US9212182, 1053)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(ccc1C)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-9.24,;,-12.32,;-1.33,-13.09,;.77,-13.65,;-.77,-10.99,)| Show InChI InChI=1S/C27H20F3N3O5S/c1-16-3-6-19(27(28,29)30)15-21(16)17-4-8-23(24(14-17)37-2)33-22-9-7-20(13-18(22)5-10-26(33)34)39(35,36)32-25-11-12-38-31-25/h3-15H,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217696
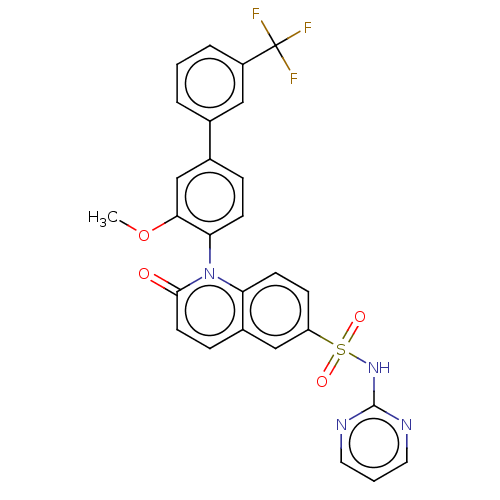
(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217799
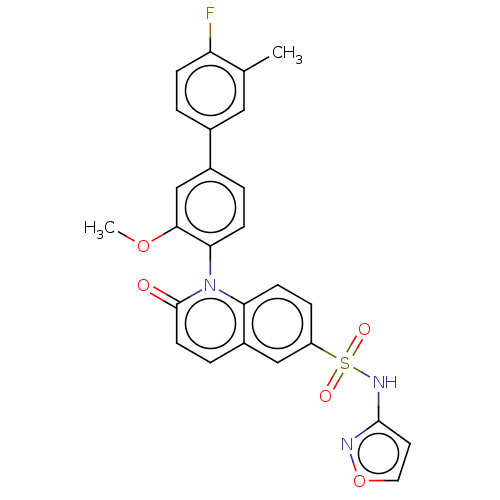
(US9212182, 1054)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H20FN3O5S/c1-16-13-17(3-7-21(16)27)18-4-8-23(24(15-18)34-2)30-22-9-6-20(14-19(22)5-10-26(30)31)36(32,33)29-25-11-12-35-28-25/h3-15H,1-2H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217725
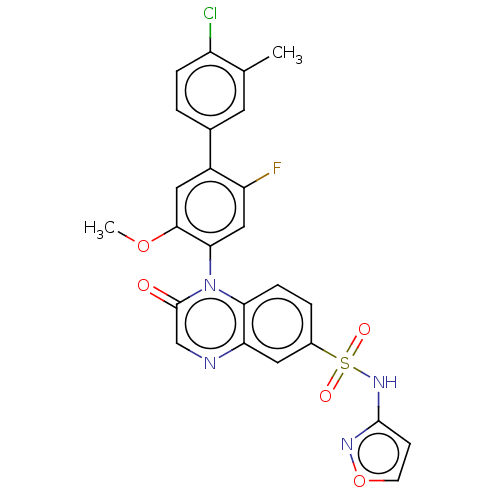
(US9212182, 652 | US9212182, 653 | US9212182, 654)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ncc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H18ClFN4O5S/c1-14-9-15(3-5-18(14)26)17-11-23(35-2)22(12-19(17)27)31-21-6-4-16(10-20(21)28-13-25(31)32)37(33,34)30-24-7-8-36-29-24/h3-13H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217447
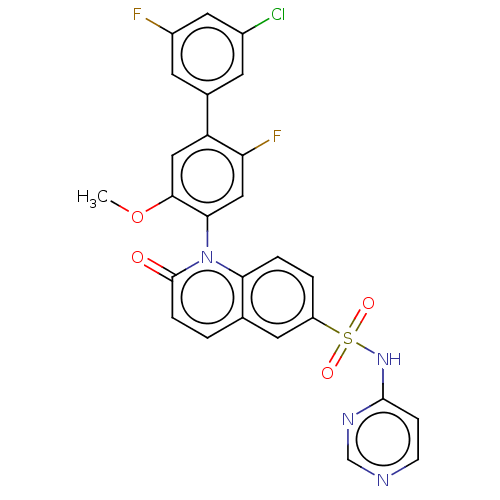
(US9212182, 540 | US9212182, 541)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccncn1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H17ClF2N4O4S/c1-37-24-12-20(16-8-17(27)11-18(28)9-16)21(29)13-23(24)33-22-4-3-19(10-15(22)2-5-26(33)34)38(35,36)32-25-6-7-30-14-31-25/h2-14H,1H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217465
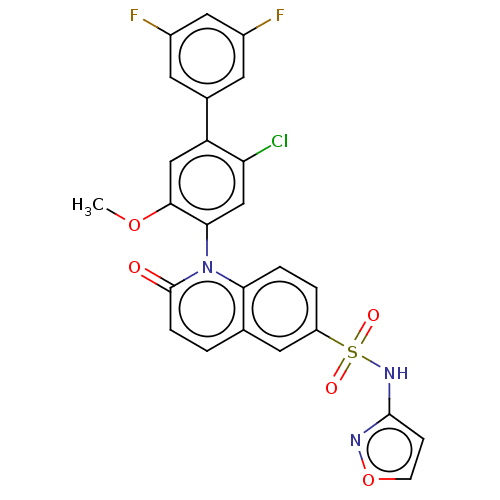
(US9212182, 457 | US9212182, 458)Show SMILES COc1cc(c(Cl)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C25H16ClF2N3O5S/c1-35-23-12-19(15-8-16(27)11-17(28)9-15)20(26)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217481
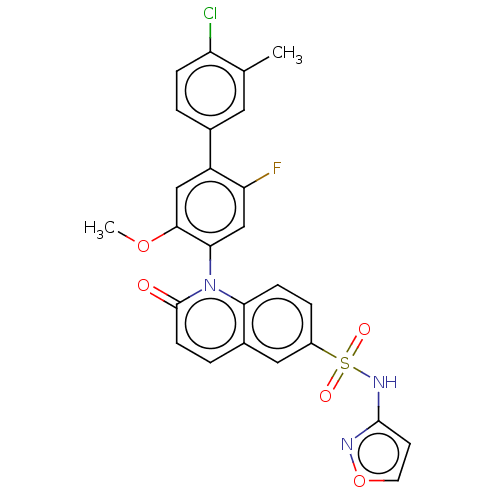
(US9212182, 469 | US9212182, 470)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H19ClFN3O5S/c1-15-11-16(3-6-20(15)27)19-13-24(35-2)23(14-21(19)28)31-22-7-5-18(12-17(22)4-8-26(31)32)37(33,34)30-25-9-10-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217656
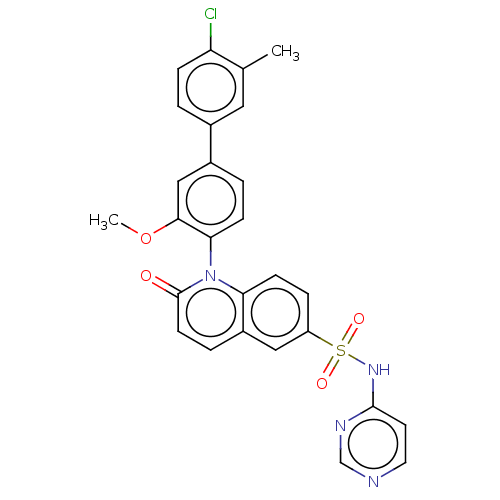
(US9212182, 658)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccncn1)-c1ccc(Cl)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H21ClN4O4S/c1-17-13-18(3-7-22(17)28)19-4-8-24(25(15-19)36-2)32-23-9-6-21(14-20(23)5-10-27(32)33)37(34,35)31-26-11-12-29-16-30-26/h3-16H,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217422

(US9212182, 419 | US9212182, 420)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;6.1,-10.99,;4.56,-13.65,)| Show InChI InChI=1S/C27H20F3N3O5S/c1-16-12-23(24(37-2)15-21(16)17-4-3-5-19(13-17)27(28,29)30)33-22-8-7-20(14-18(22)6-9-26(33)34)39(35,36)32-25-10-11-38-31-25/h3-15H,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217457
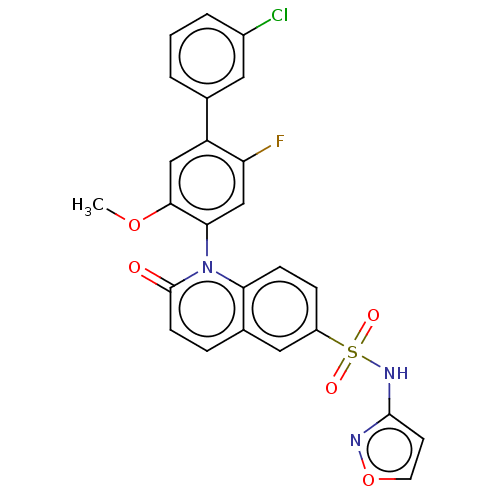
(US9212182, 475 | US9212182, 476)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H17ClFN3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217548
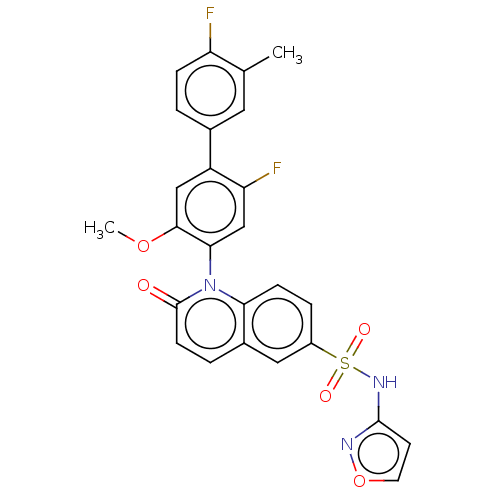
(US9212182, 512 | US9212182, 513)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H19F2N3O5S/c1-15-11-16(3-6-20(15)27)19-13-24(35-2)23(14-21(19)28)31-22-7-5-18(12-17(22)4-8-26(31)32)37(33,34)30-25-9-10-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217758

(US9212182, 696)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H20ClFN4O4S/c1-16-12-17(4-7-21(16)28)20-14-25(37-2)24(15-22(20)29)33-23-8-6-19(13-18(23)5-9-26(33)34)38(35,36)32-27-30-10-3-11-31-27/h3-15H,1-2H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50237989

(CHEMBL4084372)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(45.59,-10.99,;44.26,-11.77,;44.27,-13.31,;45.6,-14.07,;45.61,-15.62,;44.28,-16.39,;44.29,-17.93,;42.95,-15.63,;42.94,-14.09,;41.61,-13.33,;41.6,-11.78,;42.92,-11.01,;42.92,-9.48,;41.58,-8.72,;40.26,-9.49,;40.27,-11.01,;38.94,-11.78,;38.94,-13.33,;40.27,-14.1,;40.27,-15.64,;41.57,-7.17,;40.08,-6.77,;41.17,-5.68,;42.9,-6.4,;42.9,-4.86,;41.65,-3.96,;42.13,-2.49,;43.67,-2.49,;44.15,-3.96,;46.95,-16.38,;46.96,-17.92,;48.29,-18.69,;49.62,-17.91,;50.96,-18.67,;49.61,-16.36,;50.94,-15.58,;48.27,-15.61,)| Show InChI InChI=1S/C27H22FN3O5S/c1-16-13-24(25(35-3)15-21(16)18-4-7-22(28)17(2)12-18)31-23-8-6-20(14-19(23)5-9-27(31)32)37(33,34)30-26-10-11-36-29-26/h4-15H,1-3H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217829
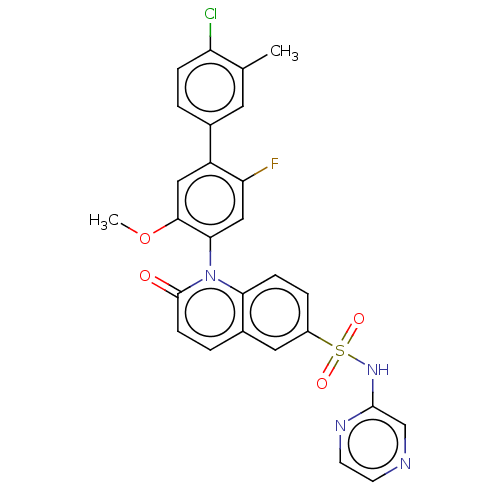
(US9212182, 691)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1cnccn1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H20ClFN4O4S/c1-16-11-17(3-6-21(16)28)20-13-25(37-2)24(14-22(20)29)33-23-7-5-19(12-18(23)4-8-27(33)34)38(35,36)32-26-15-30-9-10-31-26/h3-15H,1-2H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217517
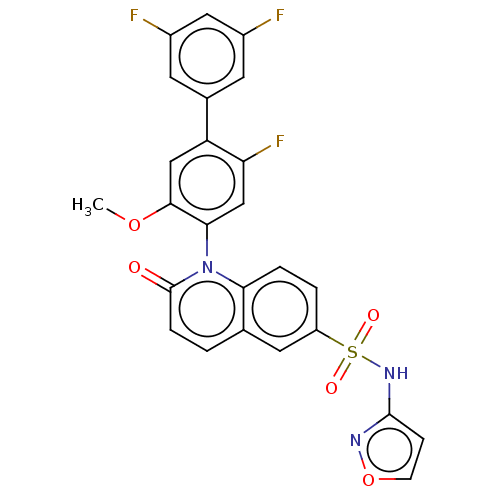
(US9212182, 363 | US9212182, 364)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C25H16F3N3O5S/c1-35-23-12-19(15-8-16(26)11-17(27)9-15)20(28)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217817

(US9212182, 683)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1ccc(Cl)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H21ClN4O4S/c1-17-14-18(4-8-22(17)28)19-5-9-24(25(16-19)36-2)32-23-10-7-21(15-20(23)6-11-26(32)33)37(34,35)31-27-29-12-3-13-30-27/h3-16H,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217889

(US9212182, 632)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(OC(F)F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;5.33,-13.86,;6.67,-14.63,;4,-14.63,;4,-10.01,)| Show InChI InChI=1S/C26H18F3N3O6S/c1-36-23-13-19(15-3-2-4-17(11-15)38-26(28)29)20(27)14-22(23)32-21-7-6-18(12-16(21)5-8-25(32)33)39(34,35)31-24-9-10-37-30-24/h2-14,26H,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217872

(US9212182, 673)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(cc1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;2.67,-12.32,;1.33,-11.55,;1.33,-10.01,;2.67,-13.86,;2.67,-15.4,;1.13,-13.86,;4.21,-13.86,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-19(15-2-5-17(6-3-15)26(28,29)30)20(27)14-22(23)33-21-8-7-18(12-16(21)4-9-25(33)34)39(35,36)32-24-10-11-38-31-24/h2-14H,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 1-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217696

(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217431
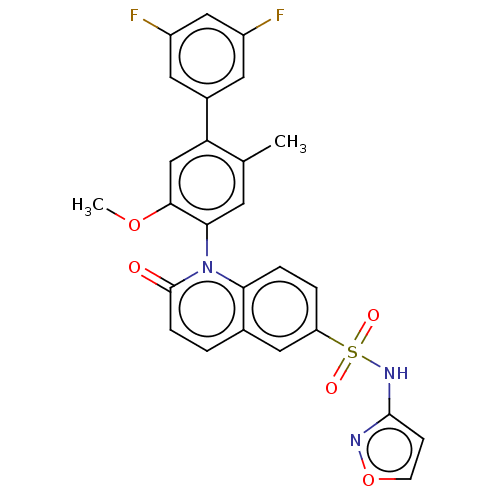
(US9212182, 423 | US9212182, 424)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C26H19F2N3O5S/c1-15-9-23(24(35-2)14-21(15)17-10-18(27)13-19(28)11-17)31-22-5-4-20(12-16(22)3-6-26(31)32)37(33,34)30-25-7-8-36-29-25/h3-14H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217435

(US9212182, 323 | US9212182, 324)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;6.1,-10.99,;4.56,-13.65,)| Show InChI InChI=1S/C26H18F3N3O5S/c1-36-23-15-17(16-3-2-4-19(13-16)26(27,28)29)5-8-22(23)32-21-9-7-20(14-18(21)6-10-25(32)33)38(34,35)31-24-11-12-37-30-24/h2-15H,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217819

(US9212182, 685)Show SMILES COc1cc(ccc1Cl)-c1cc(OC)c(cc1F)-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1 |(6.67,-11.55,;5.33,-12.32,;4,-11.55,;4,-10.01,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;,-4.62,;-1.33,-5.39,;2.67,-4.62,;4,-5.39,;4,-6.93,;5.33,-7.7,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,)| Show InChI InChI=1S/C27H20ClFN4O5S/c1-37-24-13-16(4-7-20(24)28)19-14-25(38-2)23(15-21(19)29)33-22-8-6-18(12-17(22)5-9-26(33)34)39(35,36)32-27-30-10-3-11-31-27/h3-15H,1-2H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217426
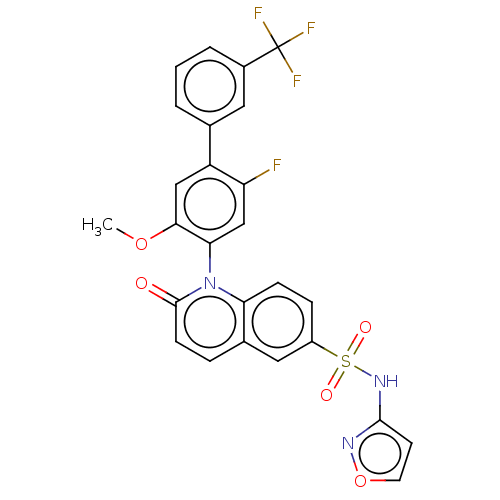
(US9212182, 359 | US9212182, 360)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-19(15-3-2-4-17(11-15)26(28,29)30)20(27)14-22(23)33-21-7-6-18(12-16(21)5-8-25(33)34)39(35,36)32-24-9-10-38-31-24/h2-14H,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217426

(US9212182, 359 | US9212182, 360)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-19(15-3-2-4-17(11-15)26(28,29)30)20(27)14-22(23)33-21-7-6-18(12-16(21)5-8-25(33)34)39(35,36)32-24-9-10-38-31-24/h2-14H,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217818
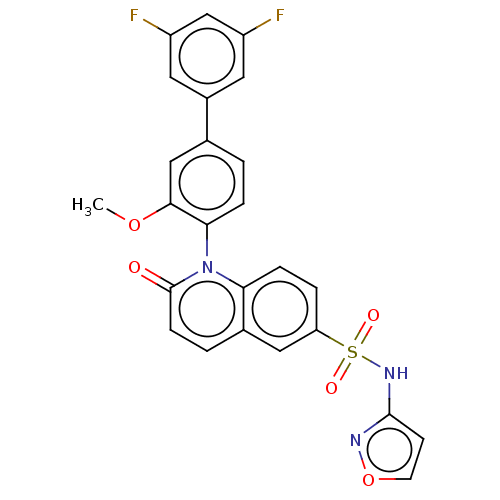
(US9212182, 684)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(F)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;4,-10.01,;4,-11.55,;5.33,-12.32,;2.67,-12.32,;1.33,-11.55,;,-12.32,;1.33,-10.01,)| Show InChI InChI=1S/C25H17F2N3O5S/c1-34-23-13-15(17-10-18(26)14-19(27)11-17)2-5-22(23)30-21-6-4-20(12-16(21)3-7-25(30)31)36(32,33)29-24-8-9-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM342945
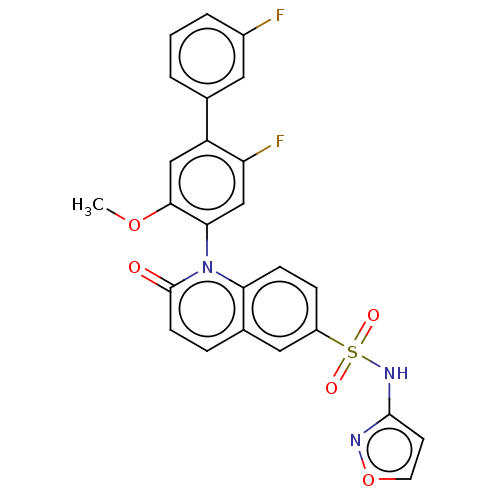
(1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isox...)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(F)c1 |(-.11,-.1,;1.22,-.87,;2.55,-.1,;2.55,1.44,;3.89,2.21,;5.22,1.44,;6.55,2.21,;5.22,-.1,;3.89,-.87,;3.89,-2.41,;2.55,-3.18,;1.22,-2.41,;-.11,-3.18,;-.11,-4.72,;1.22,-5.49,;2.55,-4.72,;3.89,-5.49,;5.22,-4.72,;5.22,-3.18,;6.55,-2.41,;-1.45,-5.49,;-2.22,-4.16,;-.68,-6.83,;-2.78,-6.26,;-4.12,-5.49,;-5.52,-6.12,;-6.55,-4.98,;-5.78,-3.64,;-4.28,-3.96,;3.89,3.75,;5.22,4.52,;5.22,6.06,;3.89,6.83,;2.55,6.06,;1.22,6.83,;2.55,4.52,)| Show InChI InChI=1S/C25H17F2N3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM342945

(1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isox...)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(F)c1 |(-.11,-.1,;1.22,-.87,;2.55,-.1,;2.55,1.44,;3.89,2.21,;5.22,1.44,;6.55,2.21,;5.22,-.1,;3.89,-.87,;3.89,-2.41,;2.55,-3.18,;1.22,-2.41,;-.11,-3.18,;-.11,-4.72,;1.22,-5.49,;2.55,-4.72,;3.89,-5.49,;5.22,-4.72,;5.22,-3.18,;6.55,-2.41,;-1.45,-5.49,;-2.22,-4.16,;-.68,-6.83,;-2.78,-6.26,;-4.12,-5.49,;-5.52,-6.12,;-6.55,-4.98,;-5.78,-3.64,;-4.28,-3.96,;3.89,3.75,;5.22,4.52,;5.22,6.06,;3.89,6.83,;2.55,6.06,;1.22,6.83,;2.55,4.52,)| Show InChI InChI=1S/C25H17F2N3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM217696

(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse Nav1.7 expressed in HEK293 cells measured after 3 to 5 mins by patchXpress electrophysiology assay |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM342945

(1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isox...)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(F)c1 |(-.11,-.1,;1.22,-.87,;2.55,-.1,;2.55,1.44,;3.89,2.21,;5.22,1.44,;6.55,2.21,;5.22,-.1,;3.89,-.87,;3.89,-2.41,;2.55,-3.18,;1.22,-2.41,;-.11,-3.18,;-.11,-4.72,;1.22,-5.49,;2.55,-4.72,;3.89,-5.49,;5.22,-4.72,;5.22,-3.18,;6.55,-2.41,;-1.45,-5.49,;-2.22,-4.16,;-.68,-6.83,;-2.78,-6.26,;-4.12,-5.49,;-5.52,-6.12,;-6.55,-4.98,;-5.78,-3.64,;-4.28,-3.96,;3.89,3.75,;5.22,4.52,;5.22,6.06,;3.89,6.83,;2.55,6.06,;1.22,6.83,;2.55,4.52,)| Show InChI InChI=1S/C25H17F2N3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217701
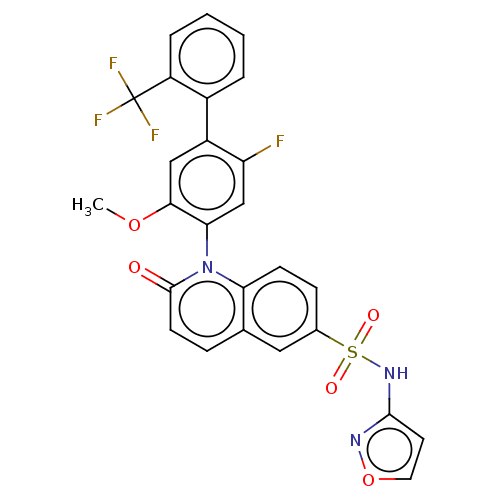
(US9212182, 955)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccccc1C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;4,-10.01,;4,-11.55,;2.67,-12.32,;1.33,-11.55,;1.33,-10.01,;,-9.24,;-1.33,-8.47,;-.77,-10.57,;.77,-7.91,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-18(17-4-2-3-5-19(17)26(28,29)30)20(27)14-22(23)33-21-8-7-16(12-15(21)6-9-25(33)34)39(35,36)32-24-10-11-38-31-24/h2-14H,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217656

(US9212182, 658)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccncn1)-c1ccc(Cl)c(C)c1 |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H21ClN4O4S/c1-17-13-18(3-7-22(17)28)19-4-8-24(25(15-19)36-2)32-23-9-6-21(14-20(23)5-10-27(32)33)37(34,35)31-26-11-12-29-16-30-26/h3-16H,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate in presence of NADPH |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM231435

(US9346798, 182)Show SMILES COc1cc(ccc1N1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O4S2/c1-28-16-10-12(19(20,21)22)2-4-14(16)25-7-8-29-17-11-13(3-5-15(17)25)31(26,27)24-18-23-6-9-30-18/h2-6,9-11H,7-8H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50237990
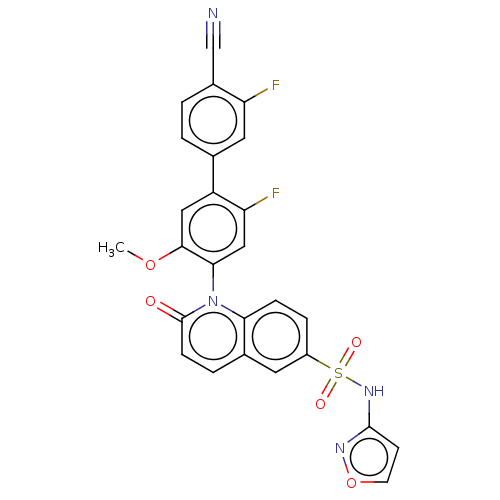
(CHEMBL4069051)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(C#N)c(F)c1 |(11.87,-30.75,;10.54,-31.53,;10.55,-33.07,;11.89,-33.83,;11.9,-35.38,;10.57,-36.15,;10.57,-37.7,;9.23,-35.39,;9.23,-33.85,;7.89,-33.09,;7.88,-31.54,;9.21,-30.77,;9.2,-29.24,;7.87,-28.47,;6.55,-29.24,;6.55,-30.77,;5.22,-31.54,;5.22,-33.09,;6.55,-33.86,;6.55,-35.4,;7.85,-26.93,;6.36,-26.52,;7.46,-25.43,;9.19,-26.15,;9.18,-24.61,;7.93,-23.72,;8.4,-22.25,;9.94,-22.24,;10.42,-23.7,;13.24,-36.14,;13.24,-37.69,;14.58,-38.45,;15.91,-37.67,;17.25,-38.43,;18.59,-39.19,;15.9,-36.12,;17.23,-35.34,;14.56,-35.37,)| Show InChI InChI=1S/C26H16F2N4O5S/c1-36-24-12-19(15-2-3-17(14-29)20(27)11-15)21(28)13-23(24)32-22-6-5-18(10-16(22)4-7-26(32)33)38(34,35)31-25-8-9-37-30-25/h2-13H,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM231476
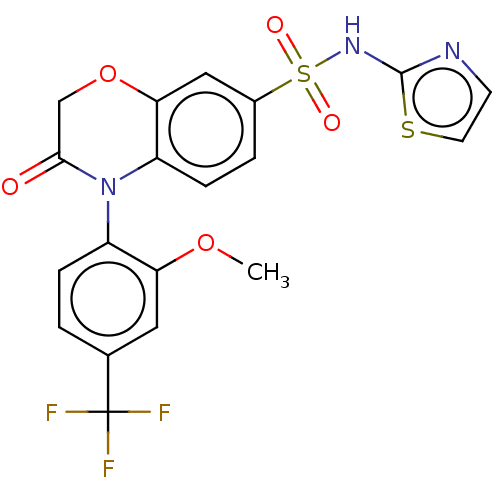
(US9346798, 223)Show SMILES COc1cc(ccc1N1C(=O)COc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O5S2/c1-29-15-8-11(19(20,21)22)2-4-13(15)25-14-5-3-12(9-16(14)30-10-17(25)26)32(27,28)24-18-23-6-7-31-18/h2-9H,10H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217458
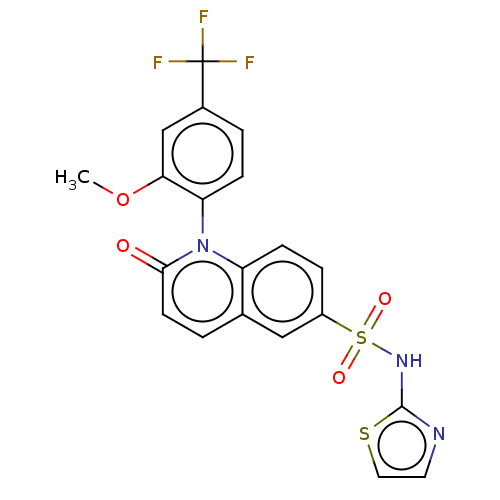
(US9212182, 463 | US9212182, 464)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1nccs1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;2.67,-10.78,;1.13,-9.24,;4.21,-9.24,)| Show InChI InChI=1S/C20H14F3N3O4S2/c1-30-17-11-13(20(21,22)23)3-5-16(17)26-15-6-4-14(10-12(15)2-7-18(26)27)32(28,29)25-19-24-8-9-31-19/h2-11H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM231435

(US9346798, 182)Show SMILES COc1cc(ccc1N1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O4S2/c1-28-16-10-12(19(20,21)22)2-4-14(16)25-7-8-29-17-11-13(3-5-15(17)25)31(26,27)24-18-23-6-9-30-18/h2-6,9-11H,7-8H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate in presence of NADPH |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217463
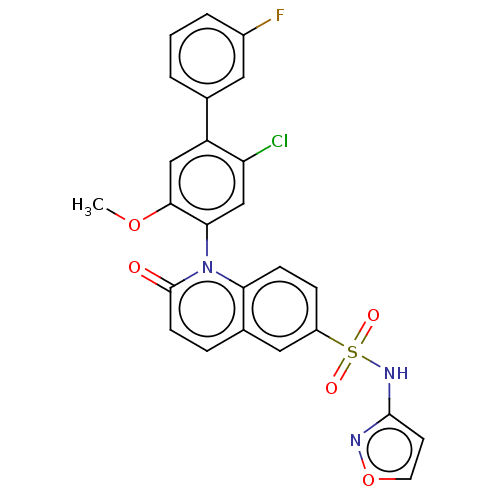
(US9212182, 455 | US9212182, 456)Show SMILES COc1cc(c(Cl)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H17ClFN3O5S/c1-34-23-13-19(15-3-2-4-17(27)11-15)20(26)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells at holding potential yielding 20 to 50% inactivation measured after 3 to 5 mins by patchXpress e... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217458

(US9212182, 463 | US9212182, 464)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1nccs1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;2.67,-10.78,;1.13,-9.24,;4.21,-9.24,)| Show InChI InChI=1S/C20H14F3N3O4S2/c1-30-17-11-13(20(21,22)23)3-5-16(17)26-15-6-4-14(10-12(15)2-7-18(26)27)32(28,29)25-19-24-8-9-31-19/h2-11H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217458

(US9212182, 463 | US9212182, 464)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1nccs1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;2.67,-10.78,;1.13,-9.24,;4.21,-9.24,)| Show InChI InChI=1S/C20H14F3N3O4S2/c1-30-17-11-13(20(21,22)23)3-5-16(17)26-15-6-4-14(10-12(15)2-7-18(26)27)32(28,29)25-19-24-8-9-31-19/h2-11H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 1-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217701

(US9212182, 955)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccccc1C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;4,-10.01,;4,-11.55,;2.67,-12.32,;1.33,-11.55,;1.33,-10.01,;,-9.24,;-1.33,-8.47,;-.77,-10.57,;.77,-7.91,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-18(17-4-2-3-5-19(17)26(28,29)30)20(27)14-22(23)33-21-8-7-16(12-15(21)6-9-25(33)34)39(35,36)32-24-10-11-38-31-24/h2-14H,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM342945

(1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isox...)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(F)c1 |(-.11,-.1,;1.22,-.87,;2.55,-.1,;2.55,1.44,;3.89,2.21,;5.22,1.44,;6.55,2.21,;5.22,-.1,;3.89,-.87,;3.89,-2.41,;2.55,-3.18,;1.22,-2.41,;-.11,-3.18,;-.11,-4.72,;1.22,-5.49,;2.55,-4.72,;3.89,-5.49,;5.22,-4.72,;5.22,-3.18,;6.55,-2.41,;-1.45,-5.49,;-2.22,-4.16,;-.68,-6.83,;-2.78,-6.26,;-4.12,-5.49,;-5.52,-6.12,;-6.55,-4.98,;-5.78,-3.64,;-4.28,-3.96,;3.89,3.75,;5.22,4.52,;5.22,6.06,;3.89,6.83,;2.55,6.06,;1.22,6.83,;2.55,4.52,)| Show InChI InChI=1S/C25H17F2N3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate in presence of NADPH |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM342945

(1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isox...)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(F)c1 |(-.11,-.1,;1.22,-.87,;2.55,-.1,;2.55,1.44,;3.89,2.21,;5.22,1.44,;6.55,2.21,;5.22,-.1,;3.89,-.87,;3.89,-2.41,;2.55,-3.18,;1.22,-2.41,;-.11,-3.18,;-.11,-4.72,;1.22,-5.49,;2.55,-4.72,;3.89,-5.49,;5.22,-4.72,;5.22,-3.18,;6.55,-2.41,;-1.45,-5.49,;-2.22,-4.16,;-.68,-6.83,;-2.78,-6.26,;-4.12,-5.49,;-5.52,-6.12,;-6.55,-4.98,;-5.78,-3.64,;-4.28,-3.96,;3.89,3.75,;5.22,4.52,;5.22,6.06,;3.89,6.83,;2.55,6.06,;1.22,6.83,;2.55,4.52,)| Show InChI InChI=1S/C25H17F2N3O5S/c1-34-23-13-19(15-3-2-4-17(26)11-15)20(27)14-22(23)30-21-7-6-18(12-16(21)5-8-25(30)31)36(32,33)29-24-9-10-35-28-24/h2-14H,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate in presence of NADPH |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217426

(US9212182, 359 | US9212182, 360)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-19(15-3-2-4-17(11-15)26(28,29)30)20(27)14-22(23)33-21-7-6-18(12-16(21)5-8-25(33)34)39(35,36)32-24-9-10-38-31-24/h2-14H,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Ability to activate estrogen receptor 2-mediated transcription. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217426

(US9212182, 359 | US9212182, 360)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C26H17F4N3O5S/c1-37-23-13-19(15-3-2-4-17(11-15)26(28,29)30)20(27)14-22(23)33-21-7-6-18(12-16(21)5-8-25(33)34)39(35,36)32-24-9-10-38-31-24/h2-14H,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50237989

(CHEMBL4084372)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(C)c1 |(45.59,-10.99,;44.26,-11.77,;44.27,-13.31,;45.6,-14.07,;45.61,-15.62,;44.28,-16.39,;44.29,-17.93,;42.95,-15.63,;42.94,-14.09,;41.61,-13.33,;41.6,-11.78,;42.92,-11.01,;42.92,-9.48,;41.58,-8.72,;40.26,-9.49,;40.27,-11.01,;38.94,-11.78,;38.94,-13.33,;40.27,-14.1,;40.27,-15.64,;41.57,-7.17,;40.08,-6.77,;41.17,-5.68,;42.9,-6.4,;42.9,-4.86,;41.65,-3.96,;42.13,-2.49,;43.67,-2.49,;44.15,-3.96,;46.95,-16.38,;46.96,-17.92,;48.29,-18.69,;49.62,-17.91,;50.96,-18.67,;49.61,-16.36,;50.94,-15.58,;48.27,-15.61,)| Show InChI InChI=1S/C27H22FN3O5S/c1-16-13-24(25(35-3)15-21(16)18-4-7-22(28)17(2)12-18)31-23-8-6-20(14-19(23)5-9-27(31)32)37(33,34)30-26-10-11-36-29-26/h4-15H,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor beta by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 8 subunit alpha
(Homo sapiens (Human)) | BDBM217696

(US9212182, 672)Show SMILES COc1cc(ccc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ncccn1)-c1cccc(c1)C(F)(F)F |(6.67,-5.39,;5.33,-4.62,;4,-5.39,;4,-6.93,;2.67,-7.7,;1.33,-6.93,;1.33,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;4.56,-13.65,;6.1,-10.99,)| Show InChI InChI=1S/C27H19F3N4O4S/c1-38-24-16-18(17-4-2-5-20(14-17)27(28,29)30)6-9-23(24)34-22-10-8-21(15-19(22)7-11-25(34)35)39(36,37)33-26-31-12-3-13-32-26/h2-16H,1H3,(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.6 expressed in HEK293 cells assessed as use-dependent block at pulse 26 after 3 to 8 mins by ionworks quattro electrophysiol... |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217422

(US9212182, 419 | US9212182, 420)Show SMILES COc1cc(c(C)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cccc(c1)C(F)(F)F |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;4,-11.55,;4,-10.01,;5.33,-12.32,;6.67,-13.09,;6.1,-10.99,;4.56,-13.65,)| Show InChI InChI=1S/C27H20F3N3O5S/c1-16-12-23(24(37-2)15-21(16)17-4-3-5-19(13-17)27(28,29)30)33-22-8-7-20(14-18(22)6-9-26(33)34)39(35,36)32-25-10-11-38-31-25/h3-15H,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217447

(US9212182, 540 | US9212182, 541)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccncn1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C26H17ClF2N4O4S/c1-37-24-12-20(16-8-17(27)11-18(28)9-16)21(29)13-23(24)33-22-4-3-19(10-15(22)2-5-26(33)34)38(35,36)32-25-6-7-30-14-31-25/h2-14H,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM217829

(US9212182, 691)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1cnccn1)-c1ccc(Cl)c(C)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-2.67,3.08,;-2.67,4.62,;-4,5.39,;-5.33,4.62,;-5.33,3.08,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C27H20ClFN4O4S/c1-16-11-17(3-6-21(16)28)20-13-25(37-2)24(14-22(20)29)33-23-7-5-19(12-18(23)4-8-27(33)34)38(35,36)32-26-15-30-9-10-31-26/h3-15H,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate in presence of NADPH |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data